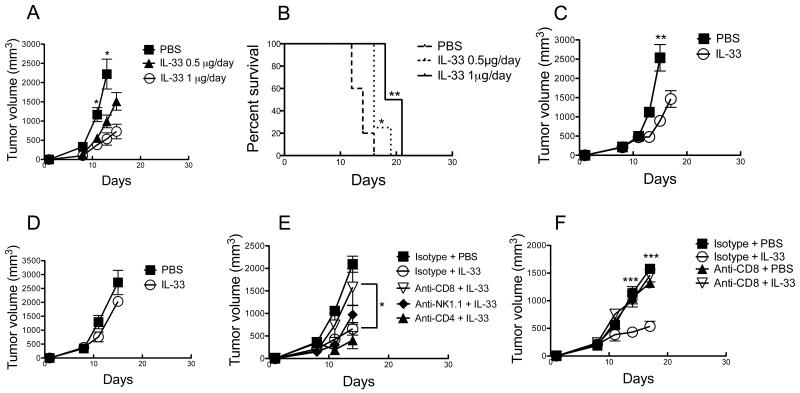
Figure 1. Administration of rIL-33 alone is sufficient to inhibit tumor growth in a CD8+ T cell dependent manner.
(A and B) The rIL-33-induced antitumor effect is dose dependent. C57BL/6 mice were injected s.c. with 106 B16-F10 cells and treated i.p. daily with the indicated dose of rIL-33 starting from d9. In (B) mice were sacrificed when tumor volume reached 2 cm3. *; p<0.05. **; p<0.01, PBS vs. 0.5 μg/day or 1 μg/day treatment. (C) Inhibition of EG7 tumor growth by rIL-33. C57BL/6 mice were injected s.c. with 106 EG7 cells and treated i.p. daily with 1 μg rIL-33 starting from d11. **; p<0.01. (D) Tumor inhibition by rIL-33 is dependent on adaptive immunity. C57BL/6 Rag1−/− mice were injected s.c. with 106 B16-F10 cells and treated i.p. daily with 1 μg rIL-33 starting from d10. (E and F) rIL-33 inhibits tumor growth in a CD8+ T cell dependent manner. Depletion of CD4+ T cells, CD8+ T cells or NK cells was achieved by twice weekly injection of anti-CD4, anti-CD8 or anti-NK1.1 depleting Abs, respectively, on the same day in EG7-bearing mice (E) or two days prior to rIL-33 or PBS administration in B16-F10-bearing mice (F). In all experiments rIL-33 per mouse was administered when tumor volume was approximately 150-250 mm3. *; p<0.05. Data (mean ± SEM) are representative of at least 3 independent experiments with 5 mice per group.