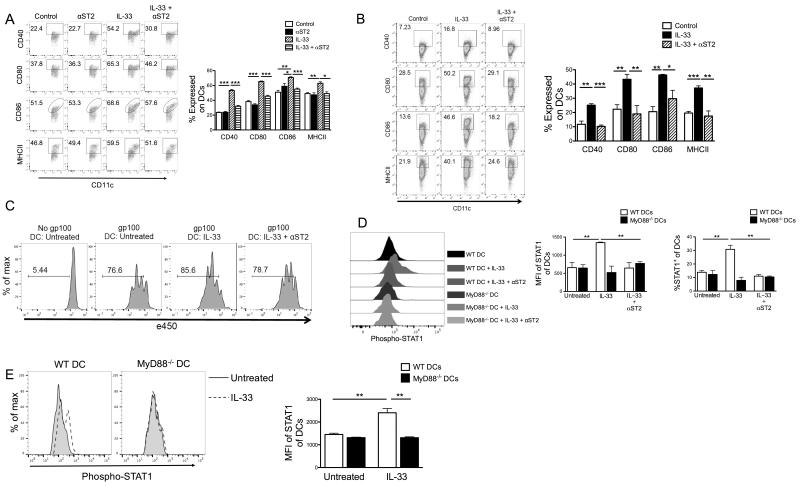
Figure 9. IL-33, ST2, MyD88 and STAT1 orchestrate activation and maturation of DCs.
Purified splenic CD11c+ cells from B16-SIY-bearing mice (A) or tumor-free mice (B) were incubated with the indicated reagents for 48 hours, and frequency of CD40, CD80, CD86, or MHC-II in Gr-1−CD11c+ cells was assessed by flow cytometry. (B) IL-33 induced maturation of naïve DCs is also ST2 dependent. (C) rIL-33-treated DCs promote antigen-specific CD8+ T cell proliferation. CD11c+ cells were treated with the indicated reagents for 48 hours as in (B), then pulsed with gp100 peptides for 2 hours. After pulsing they were cocultured with eFluro450-labeled naïve Pmel CD8+ T cells for 3 days and proliferation was measured by eFluro450 dilution. (D) rIL-33-induced STAT1 activation is ST2 and MyD88 dependent. Purified splenic WT or MyD88−/− CD11c+ cells were incubated with indicated reagents and then stained intracellularly for phosphorylated STAT1 by flow cytometry. (E) rIL-33-induced STAT1 phosphorylation is DC intrinsic. Purified CD3−CD11c+ WT and eFluro450-labeled MyD88−/− DCs were mixed at a 1:1 ratio and treated with/without rIL-33. The next day cells were analyzed for STAT1 phosphorylation by flow cytometry. WT DCs were gated as e450−, while MyD88−/− DCs were CD3−CD11c+ e450+. *; p<0.05, **; p<0.01, ***; p<0.001. Data (mean ± SEM) are representative of 3 independent experiments.