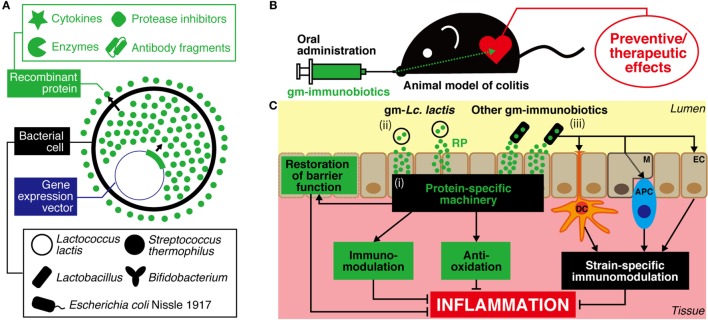
Figure 1.
A strategy for prevention and treatment of IBD using genetically modified (gm)-immunobiotics. (A) Different bioactive proteins such as cytokines, enzymes, protease inhibitors, and antibody fragments can be produced/secreted by gm-strains. (B) After oral administration, viable cells of gm-immunobiotics transit through the gastric environment and reach the intestine. Then, gm-immunobiotics provide preventive/therapeutic effects against experimental colitis in animal as a result of the exertion of anti-inflammatory effects in situ. (C) General mechanisms of action of gm-immunobiotics on anti-inflammatory effects in the intestine. Physiologically meaningful amounts of recombinant proteins are yielded by gm-immunobiotics via secretion or cell lysis, and exert host anti-inflammatory effects through a protein-specific machinery including immunomodulation, anti-oxidation, and restoration of epithelial barrier functions (i). Lactococcus (Lc.) lactis has been most widely used as a safe and effective vector in this strategy (ii). Lc. lactis has little or no effect on either the improvement or aggravation of the intestinal inflammation and does not colonize the intestine. Other gm-immunobiotics (including some strains of Lactobacillus, Bifidobacterium, and Streptococcus salivarius subsp. thermophilus, and Escherichia coli Nissle 1917) provide beneficial effects on intestinal inflammation as a result of the synergy between the immunoregulatory effects of the bacterium itself and the anti-inflammatory effects of the delivered recombinant proteins (iii). Immunobiotics interact with pattern recognition receptors of host epithelial cells and antigen-presenting cells such as dendritic cells and macrophages to exert strain-specific immunomodulatory effects. Some strains of immunobiotics may colonize the intestine. IBD, inflammatory bowel disease; RP, recombinant protein; EC, epithelial cell; M, microfold cell; DC, dendritic cell; APC, antigen-presenting cell.