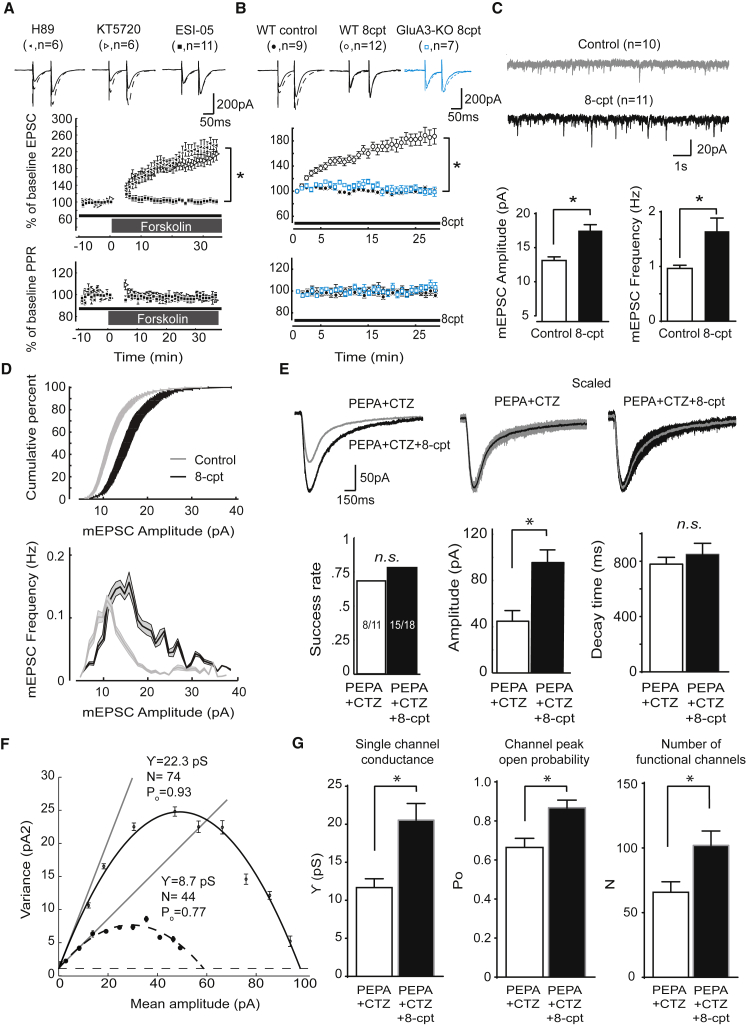
Figure 6.
GluA3 Plasticity Requires cAMP-Dependent Postsynaptic Activation of Epac
(A) Epac2 antagonist ESI-05 blocks FSK-driven synaptic potentiation, whereas PKA antagonists H89 and KT5720 do not.
(B) Intracellular application of membrane-impermeable Epac agonist 8CPT caused significant synaptic potentiation in WT PCs (open circles) compared with GluA3-KO PCs (blue boxes) or the no-drug condition in WT PCs (closed circles).
(C) Intracellular application of 8CPT caused an increase in both mEPSC amplitude (left) and frequency (right).
(D) A shift toward higher mEPSC amplitudes was visualized both in the cumulative distribution and in the mEPSC frequency-versus-amplitude distribution plots, once again suggesting postsynaptic effects of EPAC activation.
(E) Outside-out patches excised from PC somata recorded in the presence of AMPAR desensitization blockers (PEPA and CTZ) had a similar success rate of containing AMPA events (left), but generated significantly larger currents (middle) with similar decay time kinetics (right) when 8CPT was present in the internal solution.
(F) Example parabolic distribution of the variance-versus-amplitude relationship obtained from bins of the current decay profile. Nonstationary noise analysis (NSNA) was done by fitting a parabolic equation to this distribution in order to estimate conductance, open probability, and number of active receptors.
(G) NSNA performed on the PC recordings in (E) revealed significantly increased single-channel conductance (left) and peak open-channel probability upon 8CPT application (middle), which in turn led to an increased number of functional channels responding to the local AMPA application (right).
Error bars indicate SEM; ∗ indicates p < 0.05.