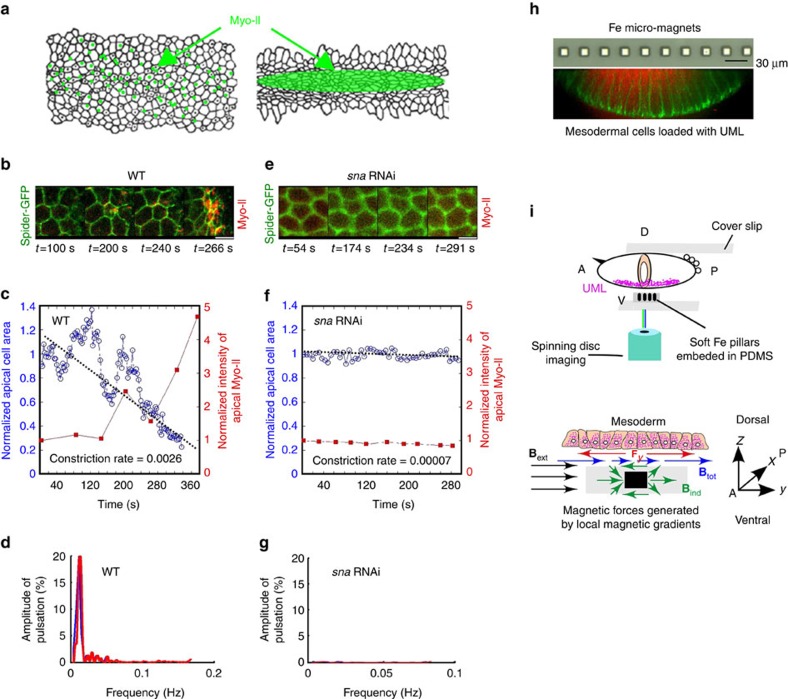
Figure 1. Use of magnetic forces to mimic sna-dependent apex pulsations in mesoderm cells.
(a) Snail-dependent stochastic fluctuation of mesoderm apex size followed by the medio-apical accumulation of Myo-II. (b) Apex size pulsations imaged in the time range 100–240 s, followed by cell constriction (image at 266 s) monitored using Spider–GFP (in green), with submembrane apical accumulation of Myo-II (sqh–mCherry in red). (c) Variation of the apical surface area (in blue) and Myo-II concentration (in red) as a function of time in the WT. Representative of the n=10 quantified on the N=64 pulsating cells, of the 587 mesoderm cells observed, in six distinct embryos. (d) Spectral signature of sna-dependent pulsations in the WT before stable constriction, following Fourier transformation of c. Representative of the n=8 cells allowing analysis (that is, staying in the same focus plane long enough) on the N=64 pulsating cells of the 587 mesoderm cells observed in six distinct embryos. (e) Absence of cell pulsations, constriction and submembrane apical accumulation of Myo-II, in sna RNAi (n=6 on N=6 embryos). (f) Variation of the apical surface area (in blue) and Myo-II concentration (in red) as a function of time in sna RNAi. Representative of the n=17 quantified cells of the 892 mesoderm cells observed in six distinct embryos. (g) Spectral signature of sna RNAi, following Fourier transformation of f. (h) Up: optical microscope image (reflection mode) of the micro-magnet network embedded in PDMS. Down: image of fluorescent UML (red) inside the basal domain of mesoderm cells, following the completion of cell formation at the end of stage 5. Green is Spider–GFP. (i) Up: schematic of the confocal-spinning disk microscope set-up in which the UML-loaded embryo is positioned on top of a transparent PDMS membrane containing a linear array of micro-magnets. Down: schematic of the homogeneous external magnetic field, Bext (in black) used to magnetize the soft micro-magnets, producing an inhomogeneous induced field, Bind (in green). The gradient of the induced field creates an in-plane tangential repulsive force Fy (in red) on UML, magnetized by Btot (in blue). A is anterior and P is posterior. White scale bars are 5 μm.