Abstract
Background
Initiation of antiretroviral therapy (ART) and subsequent virologic suppression reduces immune activation and systemic inflammation.
Methods
We examined longitudinal changes in biomarkers of monocyte activation (sCD14, sCD163), and systemic (IL-6, hsCRP, sTNFR-I and D-dimer) and vascular (Lp-PLA2) inflammation in a subgroup (N = 100 per arm) of participants enrolled in a randomized, placebo-controlled trial comparing elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (E/C/F/TAF; TAF) to E/C/F/tenofovir disoproxil fumarate (E/C/F/TDF; TDF) in treatment-naïve adults.
Results
For 194 participants (TAF, 98; TDF, 96), baseline levels of biomarkers did not differ by treatment arm; there were no differences in biomarker values between groups at weeks 12, 24, or 48 (p > 0.05), except IL-6 at week 12 (p = 0.012). Among all participants (combining groups), there were statistically significant declines from baseline observed for D-dimer, sCD163, and sTNFR-1 by week 12 and IL-6 by week 24. The proportion of participants with Lp-LA2 levels < 200 ng per mL (p = 0.250) or hsCRP levels < 3000 mg per L (p = 0.586) was unchanged through week 48.
Conclusions
We observed equivalent declines in biomarkers of monocyte activation and systemic inflammation in treatment-naïve adults treated with TAF or TDF for 48 weeks, suggesting that TAF and TDF have equivalent impact on immune activation and inflammation.
Keywords: Inflammation, Immune activation, Biomarkers, Tenofovir alafenamide
Highlights
-
•
Persons infected with HIV continue have residual immune activation and inflammation despite virologic suppression.
-
•
Several markers of immune activation and inflammation are associated with mortality and cardiovascular disease (CVD).
-
•
Decline in these markers is equivalent with tenofovir alafenamide and tenofovir disoproxil fumarate.
Biomarkers of immune activation and inflammation are associated with non AIDS–associated comorbidities and all-cause mortality. TDF decreased inflammatory markers associated with cardiovascular risk in treatment-naïve individuals. Studies have demonstrated that TAF is as efficacious at virologic suppression as TDF, and safer regarding renal and bone toxicity, but treatment differences in decline of immune activation and inflammation have not been explored. Results of our study suggest that TAF is equivalent to TDF in reducing markers of immune activation and inflammation associated with all-cause mortality, while providing an improved renal and bone safety profile compared with TDF.
1. Introduction
Treatment with combination antiretroviral therapy (ART) has increased the lifespans of persons infected with the human immunodeficiency virus (HIV) (Antiretroviral Therapy Cohort, C, 2008, Mills et al., 2011); however, immune activation and chronic inflammation have been shown to persist in spite of suppressed viral replication (Funderburg, 2014, Deeks et al., 2013, Hunt et al., 2014, Lederman et al., 2011). Several soluble markers of immune activation and inflammation, including interleukin-6 (IL-6) (Hunt et al., 2014, Sandler et al., 2011, Longenecker et al., 2014), high-sensitivity C-reactive protein (hsCRP), and tumor necrosis factor α receptor I (sTNFR-I) (Hunt et al., 2014, Tenorio et al., 2015, Kalayjian et al., 2010) are associated with all-cause mortality (Kalayjian et al., 2010 and Kuller et al., 2008) and deaths related to cardiovascular disease (CVD) (Duprez et al., 2012) in HIV-infected individuals (Tenorio et al., 2015). Soluble markers of monocyte and macrophage activation (sCD14 or sCD163) are also associated with mortality (Hunt et al., 2014 and Sandler et al., 2011), coronary calcium levels (Longenecker et al., 2014), and unstable coronary plaques (Burdo et al., 2011) in this population. Comparing the levels of immune activation and inflammation after the initiation of different ART regimens can provide additional information about the effectiveness of the regimens beyond virologic suppression.
Antiretroviral therapy regimens most commonly include two nucleoside reverse transcription inhibitors (NRTIs) and a third drug from a different class. Tenofovir disoproxil fumarate (TDF) (National Center for Biotechnology Information, n.d.-a, CID = 6,398,764) is a potent and well-tolerated NRTI recommended by all major treatment guidelines (Panel on Antiretroviral Guidelines for Adults and Adolescen, Huldrych et al., 2014, European AIDS Clinical Society, 2015), but is also associated with greater nephrotoxicity (Hall et al., 2011, Gupta, 2008, Post et al., 2010) and bone mineral density loss (Stellbrink et al., 2010, Schafer et al., 2013, McComsey et al., 2011) than other nucleoside or nucleotide reverse transcriptase inhibitors. TDF is a prodrug that is metabolized to tenofovir (TFV), which is diphosphorylated intracellularly to its active metabolite, TFV diphosphate (TFV-DP). Higher circulating plasma TFV levels have been associated with the renal and bone effects of TDF (Van Rompay et al., 2008).
Tenofovir alafenamide (TAF) (National Center for Biotechnology Information, n.d.-b, CID = 9,574,768) is a prodrug of TFV that is more stable in plasma, allowing for a ten-fold reduction in dose and resulting in a 91% reduction in plasma TFV while achieving a four-fold increase in intracellular levels of TFV-DP (Sax et al., 2015).
A single tablet co-formulation of elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (E/C/F/TAF) demonstrated high efficacy and improved renal and bone safety across multiple populations in Phase 3 trials: treatment-naïve adults and adolescents, and in adults switching from TDF-based regimens with or without chronic kidney disease (Sax et al., 2015, Mills et al., 2011, Pozniak et al., 2016, Gaur et al., 2014). It is approved by the U.S. Food and Drug Administration, 2014 and European Medicines Agency for treatment of naïve and stably suppressed patients age 12 and older and is a recommended initial regimen in U.S. Department of Health and Human Services guidelines (U.S. Department of Health and Human Services, 2015).
Here, in this randomized, selected substudy of a treatment naïve adult trial (GS-US-292-0104 [Sax et al., 2015]), we compare the effects of E/C/F/TAF versus E/C/F/TDF, referred to as the TAF and TDF groups, respectively, on biomarkers of systemic (IL-6, hsCRP, sTNFR-I) and vascular (lipoprotein-associated phospholipase A2 [Lp-PLA2]) inflammation, coagulation (D-dimer), and soluble markers of myeloid cell activation (sCD14 and sCD163) from baseline through 48 weeks of treatment. In this exploratory analysis, we hypothesized that changes in markers of immune activation would be similar between the groups, as participants in both arms were treated with a potent, integrase-based regimen, and because there were no differences in plasma virologic suppression between the TAF and TDF arms in the parent study. Several potential contributors to chronic immune activation in ART-treated HIV infection have been identified, however, including: microbial translocation and dysbiosis, co-infections, elevations in pro-inflammatory lipid levels, and low level HIV-1 replication in tissue sites that may not be captured by measurement of HIV-1 levels in plasma (Lederman et al., 2013). The differential effects of TDF and TAF on these other drivers of immune activation are also currently not known. As new ART compounds are identified, their effects on indices of immune activation and inflammation that have been linked to morbidity and mortality (Funderburg, 2014, Lederman et al., 2013) will need to be assessed.
2. Materials and Methods
2.1. Study Design
This is a substudy (n = 200) of a randomized, double-blinded, active-controlled, non-inferiority trial of treatment-naïve adults initiating TAF vs TDF (GS-US-292-0104, N = 867, ClinicalTrials.gov number NCT01780506) (Sax et al., 2015). We randomly selected 100 participants from each arm, stratifying by baseline CD4 + and HIV-1 RNA, age, sex, and geography, and excluded participants with known cardiovascular disease or statin use at baseline or during study. This study was conducted in accordance with the Declaration of Helsinki. Central or site-specific institutional review boards or ethics committees reviewed and approved the protocol. All participants provided consent for use of storage samples for future research purposes.
2.2. Measurement of Plasma Biomarkers
Plasma levels of immune activation and inflammation were measured in the Funderburg Lab (Ohio State University, Columbus, OH) using commercially available enzyme-linked immunosorbent assay (ELISA) kits. Plasma samples were thawed at 4 °C overnight and analyzed in batch. The concentrations of sCD14, IL-6, hsCRP, sCD163, and TNFR-I (R&D Systems; Minneapolis MN), D-dimer (Asserachrom D-DI immunoassay, Diagnostica Stago, Asnieres France), and Lp-PLA2 (PLAC test ELISA kit, Diadexus Inc.; San Francisco CA) were measured using protocols provided by the manufacturers. The inter- and intra-assay variability among these assays ranged between 3.9 and 11.2% and 3.4–9.8%, respectively.
2.3. Statistical Analyses
We summarized baseline levels and percent changes from baseline at post-baseline visits, compared between treatment groups using Wilcoxon Rank Sum, and tested percent changes from baseline at post-baseline visits within treatment groups and overall using Wilcoxon Signed Rank tests. We performed an additional comparison between the two baseline HIV-1 RNA categories by Wilcoxon rank sum test. For the two markers (Lp-PLA2 and hsCRP) with accepted clinical cutoffs associated with increased cardiovascular risk (< 200 or ≥ 200 ng per mL for Lp-PLA2, (Packard et al., 2000, Koenig et al., 2006, U.S. Food and Drug Administration, 2014) < 3000 or ≥ 3000 ng per mL for hsCRP (Ridker, 2016)), we used McNemar's test to compare if the proportion of participants within each paired categorical data was different at baseline and at post-baseline visits. We also stratified by baseline HIV-1 RNA category (≤ 100,000 vs > 100,000 copies per mL) to test for differences in the percent changes in any biomarkers between treatment arms. To test equivalence between arms in terms of biomarker change from baseline to week 48, two one-sided tests (TOST) were used, and 90% confidence intervals (CI) were constructed for ratios of biomarker percent change between the two arms, with baseline clinical characteristics, demographics, and other biomarkers at baseline adjusted or not adjusted. To account for correlations among biomarkers, multivariate multiple regression (MMR) was fitted for biomarker percent change from baseline to week 48 against treatment arms, with baseline clinical characteristics, demographics, and baseline biomarkers adjusted. All seven biomarkers collectively were then compared between arms using Wilks's lambda test. The machine learning algorithm Random Forest was used to assess biomarkers' ability to differentiate treatment arms using a receiver operating characteristic (ROC) curve and variable importance analysis.
3. Results
Of the 200 participants selected for this substudy, 194 had evaluable samples (TAF: n = 98; TDF: n = 96). Baseline demographic and clinical information is provided in Table 1. Median baseline age, CD4 + cell count, and viral load for the subpopulation were similar to those for the parent study. A total of 19% (37 of 194 participants) were women, 44% (85 of 194) were non-white, and 28% (55 of 194) were smokers; the median age was 33 years. The only significant differences between groups were in body mass index (median, TAF: 24 kg per m2, TDF: 25 kg per m2; p = 0.043) and percentage of smokers (TAF: 35% [34 of 98], TDF: 22% [21 of 96]; p = 0.043). Participants had a median CD4 + cell count of 405 cells per μL and a median level of viremia of 4.7 log10 copies per mL; 25% had viral load > 100,000 copies per mL.
Table 1.
Demographics and baseline clinical characteristics.
| TAF (n = 98) | TDF (n = 96) | Total (n = 194) | p-Value | |
|---|---|---|---|---|
| Age, median (IQR) | 34 (26, 41) | 32 (27, 41) | 33 (26, 41) | 0.95 |
| Female, n (%) | 21 (21) | 16 (17) | 37 (19) | 0.40 |
| Black, n (%) | 19 (19) | 13 (14) | 32 (16) | 0.27 |
| BMI, median (IQR) | 24 (22, 27) | 25 (22, 29) | 24 (22, 28) | 0.043 |
| US region, n (%) | 48 (49) | 54 (56) | 102 (53) | 0.31 |
| CD4 count, cells per μL, median (IQR) | 402 (254, 591) | 405 (297, 521) | 405 (274, 560) | 0.82 |
| CD4 < 200 cells per μL, n (%) | 16 (16) | 13 (14) | 29 (15) | |
| HIV RNA, log10 copies per mL, median (IQR) | 4.7 (4.1, 5.0) | 4.7 (4.3, 4.9) | 4.7 (4.2, 5.0) | 0.78 |
| HIV RNA < 100,000 copies per mL, n (%) | 72 (74) | 74 (77) | 146 (75) | 0.27 |
| Smoker, n (%) | 34 (35) | 21 (22) | 55 (28) | 0.043 |
| Total cholesterol, mg per dL, median (IQR) | 163 (146, 184) | 167 (149,195) | 166 (147, 187) | 0.17 |
| LDL, mg per dL, median (IQR) | 106 (88,122) | 109 (91, 129) | 107 (89, 127) | 0.20 |
| HDL, mg per dL, median (IQR) | 46 (35, 54) | 46 (37, 54) | 46 (36, 54) | 0.67 |
| Triglycerides, mg per dL, median (IQR) | 106 (77, 153) | 108 (82, 137) | 107 (80, 145) | 0.70 |
| TC:HDL ratio, median (IQR) | 3.8 (3.1, 4.3) | 3.6 (3.2, 4.4) | 3.6 (3.2, 4.3) | 0.97 |
| eGFR by Cockcroft-Gault, median (IQR) | 120 (103, 135) | 116 (102, 139) | 118 (103, 137) | 0.95 |
| Hemoglobin, g per dL, median (IQR) | 14.3 (13.2, 15.3) | 14.6 (13.5, 15.2) | 14.4 (13.2, 15.2) | 0.39 |
| Diabetes mellitus, n (%) | 3 (3) | 3 (3) | 6 (3) | 0.98 |
| Hypertension, n (%) | 10 (10) | 16 (17) | 26 (13) | 0.19 |
| Hyperlipidemia, n (%) | 2 (2) | 4 (4) | 6 (3) | 0.39 |
Bold lettering denotes a p-value less than 0.05.
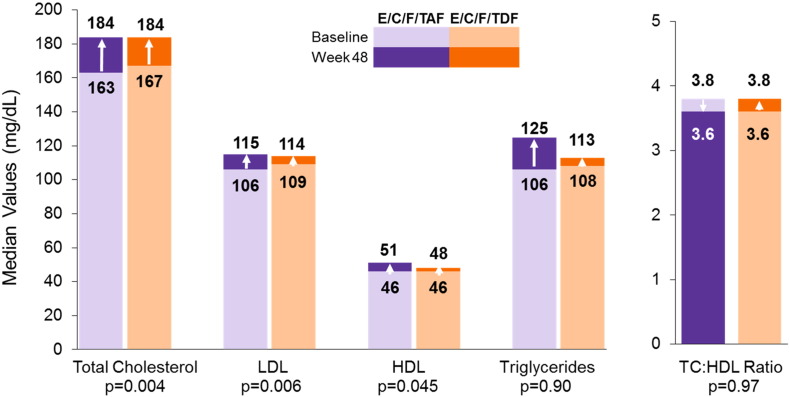
Mean increases in CD4 cell counts were similar between groups at week 48 (TAF: 240 cells per μL, TDF: 238 cells per μL; p = 0.98). Consistent with the parent study, we found similar declines in viremia between arms. A greater proportion of those receiving the TAF-containing regimen had undetectable viremia (< 50 copies per mL of HIV-1 RNA, Missing = Failure Analysis) at week 2 compared with those receiving TDF (34% [33 of 98] versus 23% [22 of 96]; p = 0.046), but this difference was not maintained through 48 weeks. From week 16 through week 48, > 95% of participants in both arms had undetectable levels of HIV-1. There were increases from baseline at week 48 in total cholesterol (TC) (TAF: 20 mg per dL; TDF: 12 mg per dL; p = 0.004), low density lipoprotein (LDL) (TAF: 12 mg per dL; TDF: 3 mg per dL; p = 0.006), high density lipoprotein (HDL) (TAF: 5 mg per dL; TDF: 3 mg per dL; p = 0.045), TC:HDL ratio (TAF: 0.1, TDF: 0.2; p = 0.97), and triglycerides (TAF: 16 mg per dL, TDF: 10 mg per dL; p = 0.90) (Fig. 1).
Fig. 1.
Fasting lipids at baseline and week 48.
P-values calculated using Wilcoxon rank-sum test for treatment comparison of change from baseline at Week 48.
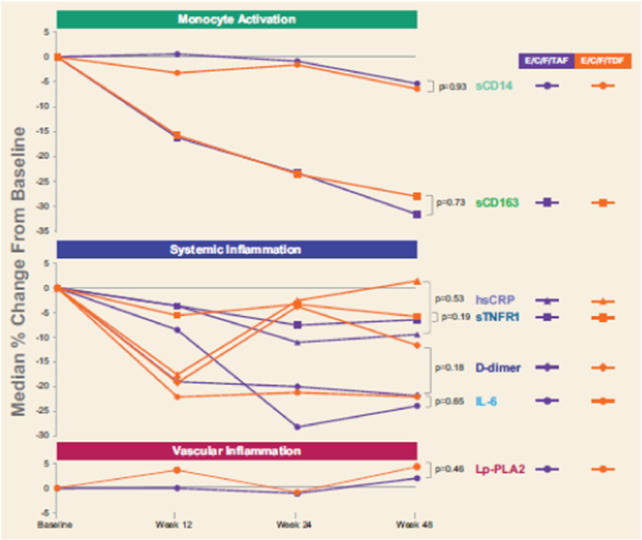
Baseline levels of biomarkers of immune activation and inflammation were similar by treatment arm, and there were no differences between treatment groups at weeks 12, 24, or 48 (p > 0.05, Supplementary Table 1) for any of the markers, except for IL-6 at week 12 only (median, TAF: 1.4 pg per mL, TDF: 1.1 pg per mL; p = 0.012). In both arms, biomarkers of systemic inflammation declined after ART initiation, with statistically significant declines (as percentage change) from baseline by week 12 for D-dimer, sCD163, and sTNFR-1 and for IL-6 by week 24 (Fig. 2, Supplementary Table 1). We did not observe a statistically significant decrease in hsCRP in either arm by 48 weeks of ART; there was a trend toward decline in sCD14 (p = 0.061). At baseline, for participants in both arms combined, the median Lp-PLA2 level was < 200 ng per mL (median [Q1, Q3] 196 [159, 23] ng per mL), indicating a low risk of cardiovascular event (Packard et al., 2000, Koenig et al., 2006, U.S. Food and Drug Administration, 2014). The proportion of participants with Lp-LA2 levels < 200 ng per mL did not change through week 48 (p = 0.250) (Supplementary Table 2). At baseline, the overall median hsCRP level was < 3000 mg per L, also indicating a low risk for a cardiovascular event (Ridker, 2016). The proportion of participants with hsCRP levels < 3000 mg per L similarly did not change through week 48 (p = 0.586). Stratifying by baseline HIV-1 RNA category (≤ 100,000 vs > 100,000 copies per mL), we also did not find significant differences in the percent changes in any biomarkers between the TAF and TDF arms at any time point. For participants in each arm and overall, we generally observed a statistically greater decline in biomarkers for those with higher baseline HIV-1 RNA (> 100,000 copies per mL) compared with those with lower baseline HIV-1 RNA (≤ 100,000 copies per mL) (Supplementary Table 3). Compared with those for participants with lower baseline viral load, the declines in D-dimer, sCD14, sCD163, and sTNF-R1 were greater for those with higher viral load at baseline at all timepoints; in IL-6 at weeks 24 and 48; and in hsCRP at week 24. We saw increases in Lp-PLA2 in the lower baseline viral load group at week 24.
Fig. 2.
Percent changes in markers of inflammation and immune activation.
Plasma samples were thawed and levels of A) soluble CD14 (sCD14), soluble CD163 (sCD163), B) C reactive protein (hsCRP), tumor necrosis factor receptor type 1 (TNFr-I), D-dimer, interleukin-6 (IL-6) and C) lipoprotein-associated phospholipase A2 (Lp-PLA2) were measured by ELISA. Differences in percent changes were assessed by Wilcoxon rank-sum test.
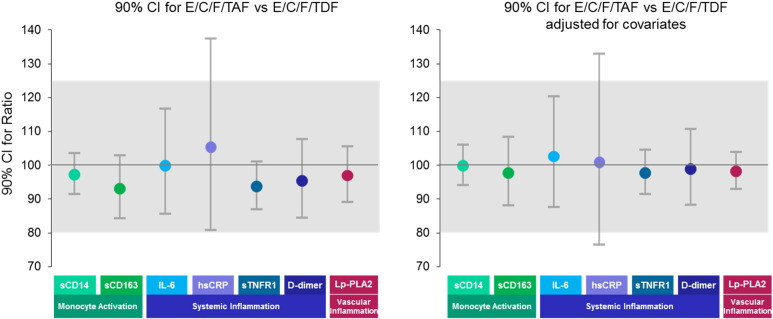
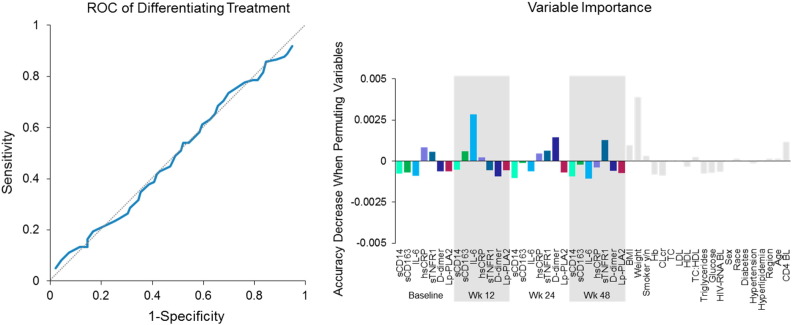
Since there was no difference between the TAF and TDF arms, we evaluated equivalence between the TAF and TDF arms using multiple statistical approaches. First, we assessed equivalence using the two one-sided test (TOST) (Fig. 3). All biomarker percent changes were equivalent between arms by TOST except hsCRP. The 90% CI of ratios between two arms were within 80% to 125% for all biomarkers, except for hsCRP, which had wider 90% CI with a ratio of 105.3% (90%CI: 80.7%, 137.4%) when not adjusting for other covariates and 100.8% (90%CI: 76.4%, 132.9%) when adjusting for other covariates. We then confirmed the equivalence finding by TOST by using MMR and Wilks's lambda test (p = 0.997); biomarker percent changes at week 48 were equivalent between arms. Lastly, we used Random Forest (ROC area under curve [AUC]), which demonstrated that there was no differentiation between arms in any biomarker percent changes at week 48 (Fig. 4). The AUC of ROC curve was 0.49 (95%CI: 0.41, 0.57), and variable importance scores were < 0.5% for all biomarkers.
Fig. 3.
Changes in levels of biomarkers: equivalence by two one-sided test.
Using TOST, equivalence is established at α significance level if a (1–2α) ∗ 100% confidence interval (CI) for the ratio of treatment 1/treatment 2 is contained within a range around 100%, such as (80%–125%).
Fig. 4.
Changes in levels of biomarkers: equivalence by Random Forest.
Machine learning algorithm Random Forest with receiver operating characteristic (ROC) curve and variable importance was used to assess the biomarkers' ability to differentiate between arms (Breiman, 2001).
BL, baseline; Hb, hemoglobin; CLcr, creatinine clearance.
4. Discussion
Initiation of ART has increased dramatically the expected lifespans of persons living with HIV; yet in spite of reducing viral replication below the limits of detection by standard clinical assays, residual chronic immune activation and systemic inflammation often persist (Deeks et al., 2013, Hunt et al., 2014, and Lederman et al., 2013, Günthard et al., 2016, European AIDS Clinical Society (EACS), 2015). As new ART drugs become available, clinicians and patients may decide whether they wish to change the current ART regimen. The decision to switch a virologically suppressive regimen has been determined by patient tolerability, pill burden/dosing frequency, and drug-associated toxicities (Panel on Antiretroviral Guidelines for Adults and Adolescents, 2015). As newer ART drugs become available with improved tolerability, clinicians and patients may wish to consider the role or association of residual immune activation and chronic inflammation and the association with non-AIDS related (e.g. non-infectious, cardiovascular) morbidity and mortality. Several contributors to chronic immune activation in ART-treated HIV infection have been identified, including microbial translocation, dysbiosis, coinfections, and low level viral replication (Lederman et al., 2013). In this substudy, we compared the effects of 48 weeks of TAF versus TDF on markers of inflammation and immune activation that have been associated with all-cause mortality and cardiovascular event risk in this population (Hunt et al., 2014, Sandler et al., 2011, Longenecker et al., 2014, Kalayjian et al., 2010, Kuller et al., 2008, Duprez et al., 2012, Burdo et al., 2011). We hypothesized that the declines in immune activation would be comparable given equivalent virologic suppression in both arms. We report that after initiation of ART regimens containing either TAF or TDF, there were equivalent declines in biomarkers of monocyte activation and systemic inflammation over 48 weeks of treatment. As expected given the results from the parent study (Sax et al., 2015), substudy participants in both arms had rapid and sustained virologic suppression and significant increases in CD4 + cell counts. Within both arms, we report significant reductions in levels of IL-6, D-dimer, sCD163, and sTNFR-1 by week 24; these decreases were sustained through week 48. Median levels of sCD14, a monocyte activation marker, declined from baseline by 5.7% by week 48, which trended toward significance (p = 0.061). Somewhat surprisingly, we did not find statistically significant reductions in plasma levels of Lp-PLA2, a marker of vascular inflammation, at any time during the study. In fact, the proportion of participants with Lp-PLA2 (as measured by mass in our study) < 200 ng per mL (the threshold for predicting cardiovascular events) remained unchanged through week 48 (p = 0.250). Of note, in a subanalysis of the JUPITER study in non-HIV infected individuals, among participants allocated to placebo, increasing quartiles of Lp-PLA2 activity (p = 0.04) but not Lp-PLA2 mass (p = 0.92) were associated with incident cardiovascular events after adjustment for LDL-C and conventional risk factors (Ridker et al., 2012).
Based on the less than expected changes in sCD14 and Lp-PLA2 in both arms, in spite of virologic control, we wanted to investigate changes in lipid levels as a potential contributor to immune activation in this study. At weeks 24 and 48, there were small, statistically significant differences in increases in total cholesterol, LDL cholesterol, and HDL cholesterol for participants receiving TAF compared with those receiving TDF, but no difference in the TC:HDL ratio, which is associated with cardiovascular risk (Deeks et al., 2013, Wilson et al., 1998, D'Agostino et al., 2008, Goff et al., 2014). These findings are consistent with what has been seen in other TAF vs TDF studies (Sax et al., 2015, Mills et al., 2015, Wohl et al., 2016) and are thought to be due to greater plasma TFV concentrations with TDF (Wohl et al., 2016, Tungsiripat et al., 2010, Santos et al., 2015, Mulligan et al., 2013, Behrens et al., 2012, Patel et al., 2014). Traditional lipid panels may underestimate cardiovascular risk in HIV-infected individuals, (Munger et al., 2015) and there is growing appreciation that pro-inflammatory lipids may contribute to immune activation in persons infected with HIV, including oxidized LDL, as levels of oxidized LDL have been associated with levels of sCD14 in this population (Zidar et al., 2015, Hileman et al., 2016, Nou et al., 2016). The basic lipid assessment performed in this substudy may not be sufficient to entirely capture changes in pro-inflammatory lipid subspecies and their contribution to persistent immune activation. In addition to the lack of change in the TC: HDL ratio, it is also reassuring, however, that there were no differences between the TAF arm and the TDF arm in Lp-PLA2 or hsCRP, indicating that the modest increases in lipid levels in the TAF arm are likely not associated with increased cardiovascular risk.
Limitations of the present analysis include that it was a post-hoc substudy and may not have been appropriately powered to be able to see differences between arms, and that we did not adjust for multiple comparisons conducted. Another potential limitation of this study is the lack of single copy assay measurement of viral replication or measurement of HIV-1 replication in the tissues. Based on the higher intracellular concentrations of TFV associated with TAF, a more sensitive assay may have identified a differential decline in viral replication between the two arms; however, as both TAF and TDF were given with the highly effective boosted integrase regimens, it may not be possible to have seen this effect, even with a more sensitive assay. Indeed, when using an HIV-1 RNA lower limit of detection of < 20 copies/mL at Week 48, no differences in the rate of viral suppression was seen between the two treatment arms (Sax et al., 2015). Also, as there are likely several contributors of immune activation and inflammation in ART-treated HIV infection, the plasma based assays we used to measure changes in immune activation indices may not be sensitive enough to detect subtle changes associated with modulation of any of these potential drivers of activation, including viral replication on single cell level. We report that after initiation of ART, regimens containing either TAF or TDF have equivalent reductions in markers of immune activation and inflammation in persons living with HIV. This is a key finding, as HIV-infected individuals are living longer, experiencing a greater cumulative exposure to ART, and are at a greater risk for treatment-associated toxicities. Treatment with a TAF-based therapy, compared to one containing TDF, has been associated with smaller declines in bone mineral density and decreased renal toxicity (Sax et al., 2015).
5. Conclusions
Treatment with TAF, compared to TDF, yields equivalent improvements in immune health and inflammatory indices, with decreased bone and renal toxicity. Beyond durable suppression of HIV viremia, treatment strategies should seek to optimize viral suppression while reducing residual immune activation and inflammation, which are increasingly associated with the clinical events that now contribute substantially to the long-term morbidity and mortality of persons living with HIV on lifelong therapy.
Funding Sources
This work was supported by Gilead Sciences.
Conflicts of Interests
NTF has received consulting and laboratory service contract fees from Gilead Sciences. GM has received grants from Gilead, BMS, ViiV, ICON, and Merck and consulting fees from Gilead, BMS, Viiv, and ICON. MK, TB, JM, and BT have received laboratory service contract fees from Gilead Sciences. HL, YZ, QS, LF, JD, AC, SM, MF, and MD are employees and stockholders of Gilead Sciences, Inc.
Author Contributors
NTF and GAM reviewed and interpreted analyses of data and edited and approved the report. NTF, MK, TB, JM, and BT performed the sample analyses and edited the draft report. HCL, TZ, QS, and LF did the data analyses, which were reviewed and interpreted by JD, AC, MWF, and MD. The first draft was written by NTF. The manuscript was edited by NTF, GAM, MK, TB, JM, BT, HCL, TZ, QS, LF, JD, AC, MWF, and MD.
Acknowledgements
The authors extend our thanks to the participants, their partners and families, and all principal investigators and their study staff who were part of the GS-US-292-0104 study. The authors also thank the complete GS-US-292-0104 study team and Anna Kido (Gilead Sciences) for providing editorial assistance.
Information in this manuscript was previously presented at CROI (2016) Conference on Retroviruses and Opportunistic Infections - 23rd Annual Conference (Feb 22, 2016; Boston, MA, USA)
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.10.009.
Appendix A. Supplementary Data
Supplementary tables
References
- Antiretroviral Therapy Cohort, C Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372(9635):293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens G., Maserati R., Rieger A. Switching to tenofovir/emtricitabine from abacavir/lamivudine in HIV-infected adults with raised cholesterol: effect on lipid profiles. Antivir. Ther. 2012;17(6):1011–1020. doi: 10.3851/IMP2305. [DOI] [PubMed] [Google Scholar]
- Breiman L. Random Forests. Mach. Learn. 2001;45(1):5–32. [Google Scholar]
- Burdo T.H., Lo J., Abbara S. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J. Infect. Dis. 2011;204(8):1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino R.B., Sr., Vasan R.S., Pencina M.J. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- Deeks S.G., Tracy R., Douek D.C. Systemic effects of inflammation on health during chronic HIV infection. Immunity. 2013;39(4):633–645. doi: 10.1016/j.immuni.2013.10.001. (2013 Oct 17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprez D.A., Neuhaus J., Kuller L.H. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One. 2012;7(9):e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European AIDS Clinical Society EACS Guideline Version 8.0 October 2015. 2015. 2015. http://www.eacsociety.org/files/guidelines_8_0-english_web.pdf [online] Available at. Accessed 01 April 2016.
- European AIDS Clinical Society (EACS) European Guidelines for treatment of HIV-positive adults in Europe, Version 8.0, 2015. 2015. http://www.eacsociety.org/files/guidelines_8.0-english-revised_20160610.pdf [online] Available at. Accessed 26 August 2016.
- Funderburg N.T. Markers of coagulation and inflammation often remain elevated in ART-treated HIV-infected patients. Curr. Opin. HIV AIDS. 2014;9(1):80–86. doi: 10.1097/COH.0000000000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur A., Fourie J., Chokephaibulkit K. 2014. Pharmacokinetics, Efficacy and Safety of an Integrase Inhibitor-Based Single-Tablet Regimen in HIV-Infected Treatment-Naive Adolescents [Poster 909]. Paper presented at: Presented at: 21st Conference on Retroviruses and Opportunistic Infections; March 3–6, 2014; Boston, MA. [Google Scholar]
- Goff D.C., Jr., Lloyd-Jones D.M., Bennett G. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. (Erratum in: Circulation. 2014 Jun 24;129(25 Suppl 2), pp. :S74–5. doi: 10.1161/01.cir.0000437741.48606.98) [DOI] [PubMed] [Google Scholar]
- Günthard H.F., Saag M.S., Benson C.A. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the international antiviral society–USA panel. JAMA. 2016;316(2):191–210. doi: 10.1001/jama.2016.8900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S.K. Tenofovir-associated Fanconi syndrome: review of the FDA adverse event reporting system. AIDS Patient Care STDs. 2008;22(2):99–103. doi: 10.1089/apc.2007.0052. [DOI] [PubMed] [Google Scholar]
- Hall A.M., Hendry B.M., Nitsch D., Connolly J.O. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am. J. Kidney Dis. 2011;57(5):773–780. doi: 10.1053/j.ajkd.2011.01.022. [DOI] [PubMed] [Google Scholar]
- Hileman C.O., Turner R., Funderburg N.T.N.T.F., Semba R.D., McComsey G.A. Changes in oxidized lipids drive the improvement in monocyte activation and vascular disease after statin therapy in HIV. AIDS. 2016;30(1):65–73. doi: 10.1097/QAD.0000000000000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huldrych F., Gunthard H.F., Aberg J.A. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the international antiviral society-USA panel. JAMA. 2014;312(4):410–425. doi: 10.1001/jama.2014.8722. [DOI] [PubMed] [Google Scholar]
- Hunt P.W., Sinclair E., Rodriguez B. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J. Infect. Dis. 2014;210(8):1228–1238. doi: 10.1093/infdis/jiu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalayjian R.C., Machekano R.N., Rizk N. Pretreatment levels of soluble cellular receptors and interleukin-6 are associated with HIV disease progression in subjects treated with highly active antiretroviral therapy. J. Infect. Dis. 2010;201(12):1796–1805. doi: 10.1086/652750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig W., Twardella D., Brenner H., Rothenbacher D. Lipoprotein-associated phospholipase A2 predicts future cardiovascular events in patients with coronary heart disease independently of traditional risk factors, markers of inflammation, renal function, and hemodynamic stress. Arterioscler. Thromb. Vasc. Biol. 2006;26(7):1586–1593. doi: 10.1161/01.ATV.0000222983.73369.c8. [DOI] [PubMed] [Google Scholar]
- Kuller L.H., Tracy R., Belloso W. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman M.M., Calabrese L., Funderburg N.T. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J. Infect. Dis. 2011;204(8):1217–1226. doi: 10.1093/infdis/jir507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman M.M., Funderburg N.T., Sekaly R.P., Klatt N.R., Hunt P.W. Residual immune dysregulation syndrome in treated HIV infection. Adv. Immunol. 2013;119:51–83. doi: 10.1016/B978-0-12-407707-2.00002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longenecker C.T., Jiang Y., Orringer C.E. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS. 2014;28(7):969–977. doi: 10.1097/QAD.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McComsey G.A., Kitch D., Daar E.S. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: aids clinical trials group A5224s, a substudy of ACTG A5202. J. Infect. Dis. 2011;203(12):1791–1801. doi: 10.1093/infdis/jir188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E.J., Bakanda C., Birungi J. Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Ann. Intern. Med. 2011;155(4):209–216. doi: 10.7326/0003-4819-155-4-201108160-00358. [DOI] [PubMed] [Google Scholar]
- Mills A., Arribas J.R., Andrade-Villanueva J. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect. Dis. 2015;16(1):43–52. doi: 10.1016/S1473-3099(15)00348-5. [DOI] [PubMed] [Google Scholar]
- Mulligan K., Glidden D.V., Anderson P.L. Presented at the 15th International Workshop on Co-morbidities and Adverse Drug Reactions in HIV. Brussels, Belgium. 2013. Decreases in cholesterol in HIV-seronegative men using emtricitabine/tenofovir pre-exposure prophylaxis: lipid results of iPrEx [Abstract 005]. 2013.https://www.intmedpress.com/serveFile.cfm?sUID=bc75b62c-c206-437a-b725-a75baa67f5b2 [online] Available at. Accessed 01 April 2016. [Google Scholar]
- Munger A.M., Chow D.C., Playford M.P. Characterization of lipid composition and high-density lipoprotein function in HIV-infected individuals on stable antiretroviral regimens. AIDS Res. Hum. Retrovir. 2015;31(2):221–228. doi: 10.1089/aid.2014.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Biotechnology Information PubChem Compound Database; CID = 6398764. https://pubchem.ncbi.nlm.nih.gov/compound/Tenofovir_Disoproxil_Fumarate#section=Top [online] Available at. [Accessed 28 August 2016]
- National Center for Biotechnology Information PubChem Compound Database; CID = 9574768. https://pubchem.ncbi.nlm.nih.gov/compound/9574768 [online] Available at. [Accessed 28 August 2016]
- Nou E., Lu M.T., Looby S.E. Serum oxidized low-density lipoprotein decreases in response to statin therapy and relates independently to reductions in coronary plaque in patients with HIV. AIDS. 2016;30(4):583–590. doi: 10.1097/QAD.0000000000000946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard C.J., O'Reilly D.S.J., Caslake M.J. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. N. Engl. J. Med. 2000;343(16):1148–1155. doi: 10.1056/NEJM200010193431603. [DOI] [PubMed] [Google Scholar]
- Panel on Antiretroviral Guidelines for Adults and Adolescents . 2015. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents; http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdfpp. 1–166. [online] Available at. [Accessed 01 April 2016] [Google Scholar]
- Patel D.A., Snedecor S.J., Tang W.Y. 48-week efficacy and safety of dolutegravir relative to commonly used third agents in treatment-naive HIV-1-infected patients: a systematic review and network meta-analysis. PLoS One. 2014;9(9):e105653. doi: 10.1371/journal.pone.0105653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post F.A., Moyle G.J., Stellbrink H.J. Randomized comparison of renal effects, efficacy, and safety with once-daily abacavir/lamivudine versus tenofovir/emtricitabine, administered with efavirenz, in antiretroviral-naive, HIV-1-infected adults: 48-week results from the ASSERT study. J. Acquir. Immune Defic. Syndr. 2010;55(1):49–57. doi: 10.1097/QAI.0b013e3181dd911e. [DOI] [PubMed] [Google Scholar]
- Pozniak A., Arribas J.R., Gathe J. Switching to tenofovir alafenamide, coformulated with elvitegravir, cobicistat, and emtricitabine, in HIV-infected patients with renal impairment: 48 week results from a single-arm, multi-center, open-label, Phase 3 study. J. Acquir. Immune Defic. Syndr. 2016;71(5):530–537. doi: 10.1097/QAI.0000000000000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker P.M. A test in context: high-sensitivity C-reactive protein. J. Am. Coll. Cardiol. 2016;67(6):712–723. doi: 10.1016/j.jacc.2015.11.037. [DOI] [PubMed] [Google Scholar]
- Ridker P.M., MacFadyen J.G., Wolfert R.L., Koenig W. Relationship of lipoprotein-associated phospholipase A₂ mass and activity with incident vascular events among primary prevention patients allocated to placebo or to statin therapy: an analysis from the JUPITER trial. Clin. Chem. 2012;58(5):877–886. doi: 10.1373/clinchem.2011.180281. [DOI] [PubMed] [Google Scholar]
- Sandler N.G., Wand H., Roque A. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J. Infect. Dis. 2011;203(6):780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos J.R., Saumoy M., Curran A. The lipid-lowering effect of tenofovir/emtricitabine: a randomized, crossover, double-blind, placebo-controlled trial. Clin. Infect. Dis. 2015;61(3):403–408. doi: 10.1093/cid/civ296. [DOI] [PubMed] [Google Scholar]
- Sax P.E., Wohl D., Yin M.T. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385(9967):2606–2615. doi: 10.1016/S0140-6736(15)60616-X. [DOI] [PubMed] [Google Scholar]
- Schafer J.J., Manlangit K., Squires K.E. Bone health and human immunodeficiency virus infection. Pharmacotherapy. 2013;33(6):665–682. doi: 10.1002/phar.1257. [DOI] [PubMed] [Google Scholar]
- Stellbrink H.J., Orkin C., Arribas J.R. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin. Infect. Dis. 2010;51(8):963–972. doi: 10.1086/656417. [DOI] [PubMed] [Google Scholar]
- Tenorio A.R., Chan E.S., Bosch R.J. Rifaximin has a marginal impact on microbial translocation, T-cell activation and inflammation in HIV-positive immune non-responders to antiretroviral therapy – ACTG A5286. J. Infect. Dis. 2015;211(5):780–790. doi: 10.1093/infdis/jiu515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tungsiripat M., Kitch D., Glesby M.J. A pilot study to determine the impact on dyslipidemia of adding tenofovir to stable background antiretroviral therapy: ACTG 5206. AIDS. 2010;24(11):1781–1784. doi: 10.1097/QAD.0b013e32833ad8b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents Includes a Fixed-Dose Combination of Elvitegravir/Cobicistat/Emtricitabine/Tenofovir Alafenamide Among the Recommended Regimens for Antiretroviral Treatment-Naive Individuals with HIV-1 Infection. 2015. https://aidsinfo.nih.gov/news/1621/evg-c-ftc-taf--statement-from-adult-arv-guideline-panel [press release]. November 18, 2015. [online] Available at. Accessed 01 April 2016.
- U.S. Food and Drug Administration . U.S. Food and Drug Administration; Silver Spring, MD: 2014. FDA Clears Test that Helps Predict the Risk of Coronary Heart Disease. [Press Release]http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm426799.htm [online] Available at. Accessed 01 April 2016. [Google Scholar]
- Van Rompay K.K., Durand-Gasselin L.A.D.-G.L., Brignolo L.L. Chronic administration of tenofovir to rhesus macaques from infancy through adulthood and pregnancy: summary of pharmacokinetics and biological and virological effects. Antimicrob. Agents Chemother. 2008;52(9):3144–3160. doi: 10.1128/AAC.00350-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson P.W., D'Agostino R.B., Levy D., Belanger A.M., Silbershatz H., Kannel W.B. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- Wohl D., Oka S., Clumeck N. Brief report: a randomized, double-blind comparison of tenofovir alafenamide versus tenofovir disoproxil fumarate, each coformulated with elvitegravir, cobicistat, and emtricitabine for initial HIV-1 treatment: week 96 results. J. Acquir. Immune Defic. Syndr. 2016;72(1):58–64. doi: 10.1097/QAI.0000000000000940. [DOI] [PubMed] [Google Scholar]
- Zidar D.A., Juchnowski S., Ferrari B. Oxidized LDL levels are increased in HIV infection and may drive monocyte activation. J. Acquir. Immune Defic. Syndr. 2015;69(2):154–160. doi: 10.1097/QAI.0000000000000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables