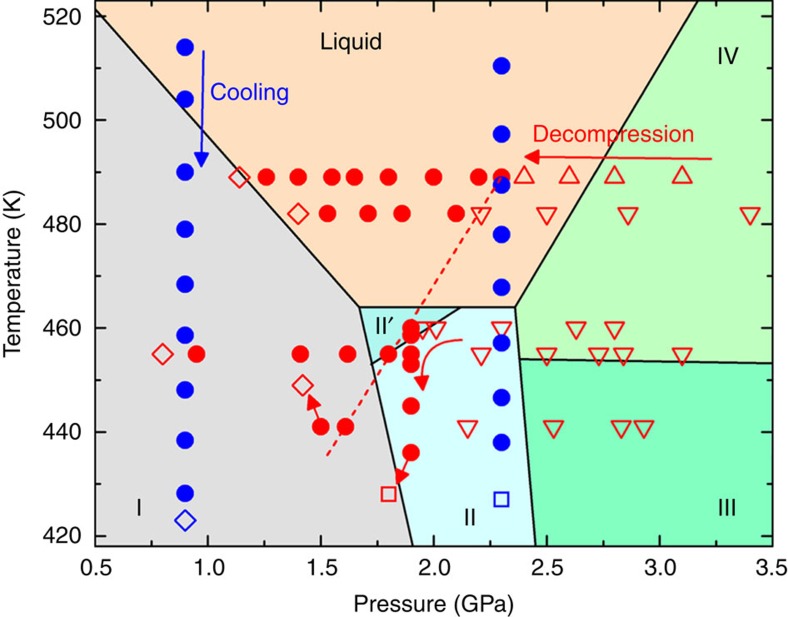
Figure 2. Experimental conditions where the metastable liquid is observed.
Bi-III′ below 489 K is obtained by compressing the DIL at 489 K, and then cooled down to lower temperatures. Under decompression (red left arrows) at 489, 482, 460, 455 and 441 K, Bi-III′ (red open down triangles) and Bi-IV (red open up triangles) melt into liquid Bi (red solid circles), followed by crystallization into Bi-I (red open diamonds). Upon compression, transition sequences of Bi-I→II (or II′)→IV (or mixture of IV+III) are observed (Supplementary Figs 2, 4, 7). Red dash line is a guide for eyes representing the decompression-induced melting line of Bi-III′ (or IV). At 460 K, Bi-III melts into the DIL at 1.9 GPa under decompression, and then the DIL is cooled (red down arrow) from 460 to 423 K at ∼1.8 GPa. The DIL crystallizes into Bi-II (red open squares) under cooling at 428 K with a slight change in pressure. At 441 K, Bi-III′ melts into liquid Bi at∼1.6 GPa. Then the DIL crystallizes into Bi-I upon heating (red up arrow) at 1.5 GPa from 441 to 449 K. Blue solid circles indicate liquid Bi under cooling (blue down arrow) from equilibrium liquid to supercooled liquid at ∼0.9 and 2.2 GPa, respectively. The supercooled liquid Bi crystallizes into Bi-I (blue open diamonds) at 0.9 GPa 423 K and into Bi-II (blue open squares) at 2.2 GPa 426 K, respectively.