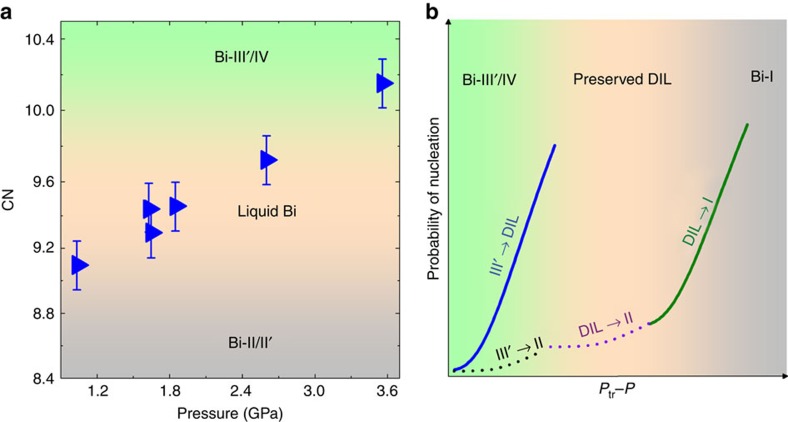
Figure 4. Coordination number of Bi in metastable liquid and probability of nucleation.
(a) Change of the nearest-neighbour coordination number (CN) of liquid Bi with pressure. The CN (blue solid right triangle) is calculated using the pair distribution function of liquid, assuming that the density of liquid is equal to that of Bi-II at corresponding pressure–temperature conditions. The uncertainties of CN are estimated from the errors in fitting the first sharp peak in the pair distribution functions of the liquid. The CN is close to that of Bi-II at low pressures, while the CN increases with pressure and approaches to that of Bi-IV at high pressures. (b) Nucleation probability in the phase transitions as a function of Ptr−P under decompression, where Ptr is thermodynamic equilibrium pressure of Bi-III′ (or IV) and II. The probability is proportional to exp[−ΔG*/kBT], where ΔG* is the free energy barrier in the formation of nucleation, and is proportional to γ3/ (ΔGV)2; γ and ΔGV are the interfacial energy and difference of Gibbs free energy between parent and new phases, which are closely correlated to structures. In the first step, Bi-III′ to DIL transition has large probability in formation of liquid (blue solid line) compared with that of Bi-II in Bi-III to Bi-II transition (black dash line), owing to much smaller interfacial energy in solid/liquid interfaces. In the second step, the large kinetic energy barrier in crystallization of the transient liquid suppresses the formation of Bi-II (purple dash line), eventually resulting in the preservation of the DIL. Upon further decompression, probability of nucleation of Bi-I (olive solid line) increases due to the increase in difference of Gibbs free energies between DIL and Bi-I.