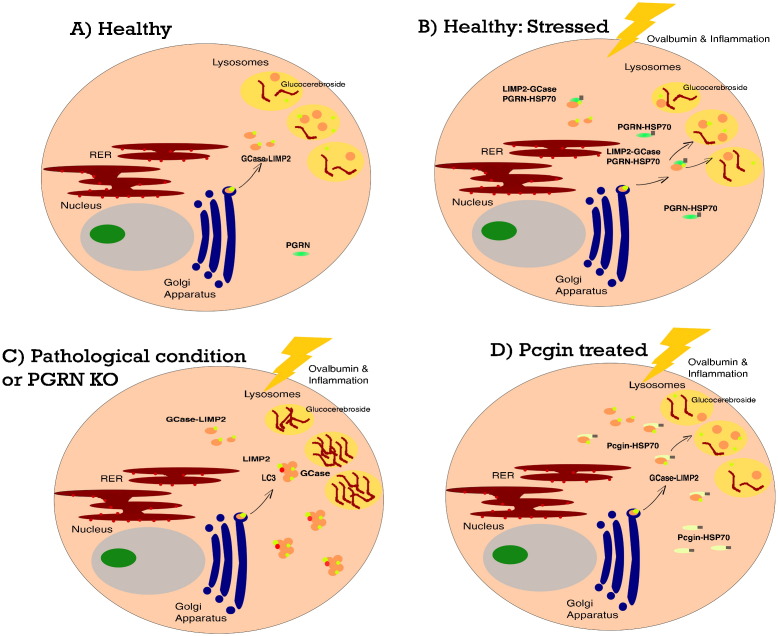
Fig. 1.
Interaction of PGRN as a co-chaperone with HSP70, GCase, and LIMP2. (A) In healthy cells, GCase binds to its receptor protein LIMP1 for lysosomal trafficking and localization. (B) When ovalbumin is used to induce stress, PGRN serves as a co-chaperone with HSP70 in the HSP70 disaggregation system that prevents GCase-LIMP1 aggregation in the cytoplasm and facilitates their trafficking/localization to the lysosome. (C) In stressed PGRN KO cells, PGRN deficiency results in GCase-LIMP1 aggregation in the cytoplasm, GCase deficiency in the lysosome, excessive glucocerebroside substrate accumulation, and cytotoxicity. (D) Pcgin treatment of PGRN KO cells and Gaucher Types 1 and 2 fibroblasts results in the delivery of GCase to the lysosome, restoration of lysosomal GCase activity, and degradation of glucocerebroside. PGRN, progranulin; HSP, heat shock protein; GCase, glucocerebrosidase; LIMP2, lysosomal integral membrane protein 2; KO, knock-out; Pcgin, progranulin C-terminus for GCase interaction.