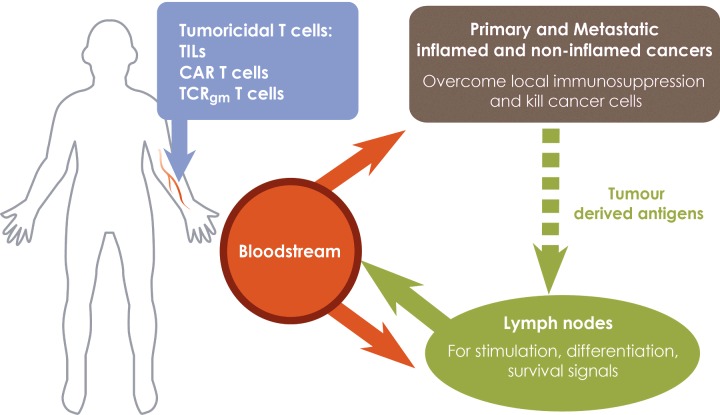
Figure 1. A designer adoptive T-cell therapy for solid cancers.
T-lymphocytes expressing conventional TCRs (TILs, TCRgm) or CARs at different stages of activation and differentiation are required to kill primary and metastatic cancers and to persist in cancer patients. Fully activated, tumouricidal T-cells expressing inflammation-associated homing molecules migrate from tumour blood vessels into primary cancers and sites of metastases (including sentinel lymph nodes) where they kill cancer cells. Tumouricidal T-cells migrating to non-cancerous tissues are unable to exert anti-cancer activity and are ineffective. Central memory T-cells expressing conventional TCRs, but not CARs, are re-activated by endogenously processed and presented tumour-derived antigens in tumour-draining lymph nodes before being redistributed to cancerous tissue. Central memory T-cells receive survival signals during normal recirculation through lymphoid organs. Recruitment of T-cells into non-inflamed cancers is promoted by patient conditioning which sensitizes the normally anergic tumour blood vessels to inflammatory mediators, increases the expression of homing-associated molecules and promotes recruitment of tumouricidal T-cells. Maturation of tumour blood vessels by activated T-cells in already inflamed tumours, promotes the development of HEV which recruit central memory T-cells into the tumour and shift the site of T-cell activation to cancerous tissues which avoids the loss of tumouricidal T-cells to non-cancerous tissues during their redistribution from the normal LN site of priming. γ-chain cytokines generate central memory and effector T-cells during T-cell expansion prior to adoptive transfer to cancer patients.