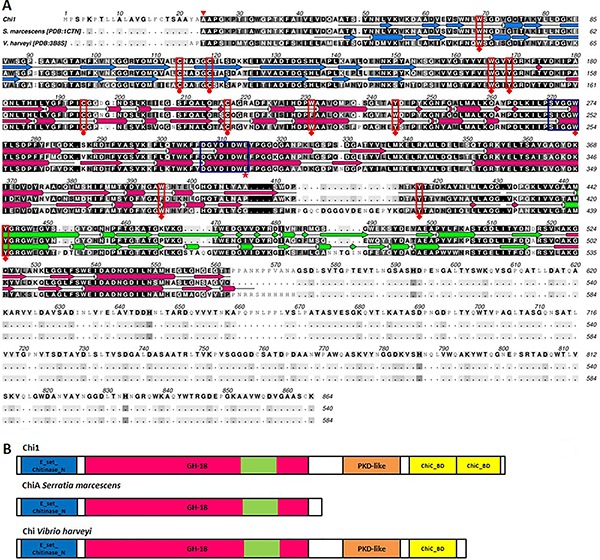
Figure 2. Amino acid sequence alignment, secondary structure estimation and domains schematic representation of MetaChi18A and most similar available chitinases structures. A, The triangle (pointing down) indicates the cleavage site of the signal peptide, diamonds indicate the conserved aromatic residues binding the substrate, circles indicate disulfide bonds and the star indicates the glutamate residue involved in the hydrolysis. Dark blue boxes indicate the conserved sequences in the catalytic domain from glycosyl hydrolases family 18. The sequence background is colored according to the convention ALSCRIPT Calcons. Representations of secondary structures are given above the sequences: arrows for β-strands and cylinders for α-helix. Light blue is the E_set_chitinase_N, N-terminal domain, pink is the catalytic domain, and green is the (α+β) insertion in catalytic domain. B, Domain representations follow the same colors with the addition of: orange - PKD (polycystic kidney disease)-like domain with Ig-like fold, which probably functions as a ligand-binding site in protein-protein or protein-carbohydrate interactions and yellow - ChiC_BD (chitin-binding domain related to ChiC of Streptomyces griseus). The N-terminal domain and the GH-18 catalytic domain from MetaChi18A [Genbank: KJ160494] have 74% identity with ChiA from Serratia marcescens [GenBank: ABI79317.1] and 54% identity with the chitinase from Vibrio harveyi [GenBank: AIV07901.1].