Figure 2. Acyl-RAC detection of SOD1 and CCS S-acylation in human spinal cords from a non-ALS and FALS patient.
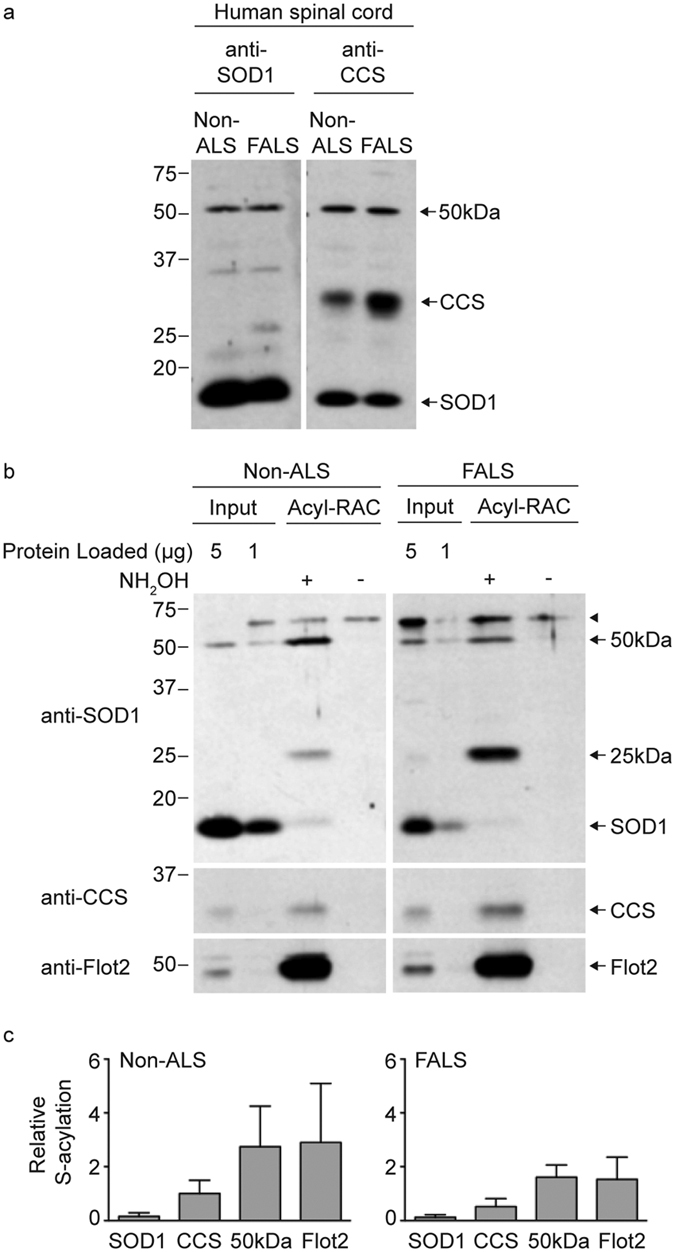
(a) Equal amounts of total protein lysates from human spinal cords were run on SDS-PAGE in duplicate and were analyzed by Western blotting with either an anti-SOD1 antibody or an anti-CCS antibody. (b) Equal amounts of total protein lysates from human spinal cords were processed for acyl-RAC. Equal amounts of acyl-RAC input protein, 100% of +NH2OH acyl-RAC bound protein, and 100% of −NH2OH acyl-RAC bound protein were analyzed by Western blotting first with an anti-SOD1 antibody and subsequently with anti-CCS and anti-flotillin-2 antibodies. (c) The densitometry values for +NH2OH bound protein signals and input protein signals were corrected for differences in exposure time and input loading. The corrected densitometry values were normalized by dividing the +NH2OH bound protein signals by the corresponding input protein signals. Graphs provide average normalized densitometry data and the error bars represent the standard error of the mean (n = 4 experiments for the SOD1 monomer, CCS monomer, and 50 kDa band; n = 2 experiments for flotillin-2). The non-ALS sample was case 2 and the SOD1 FALS sample was case 9 (see Table 1).
