Figure 3.
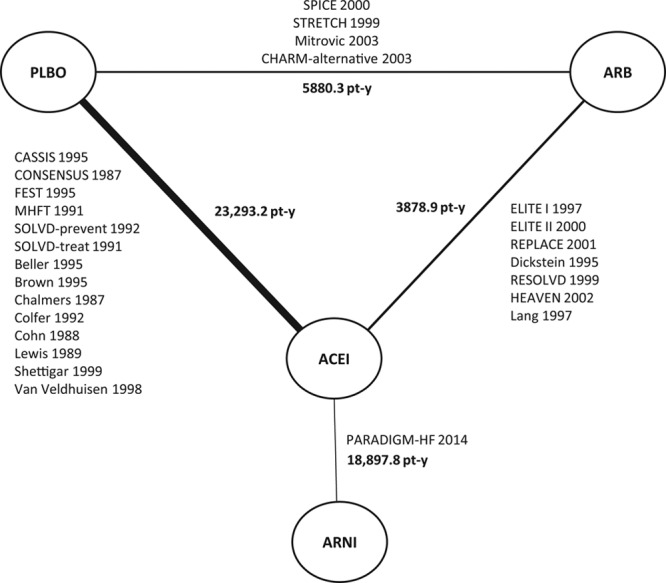
Sensitivity analysis evidence network of ARNI, ACEI, ARB and placebo for all-cause mortality ignoring background treatments. ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin-II receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; CASSIS, Czech and Slovak Spirapril Intervention Study; CHARM-alternative, Candesartan in Heart Failure–Assessment of Mortality and Morbidity Alternative; CONSENSUS, Cooperative North Scandinavian Enalapril Survival Study; ELITE, Evaluation of Losartan in the Elderly Study; FEST, Fosinopril Efficacy/Safety Trial; HEAVEN, Heart Failure Valsartan Exercise Capacity Evaluation; MHFT, Munich Mild Heart Failure Trial; PARADIGM-HF, Prospective Comparison of ARNI (Angiotensin Receptor–Neprilysin Inhibitor) With ACEI (Angiotensin–Converting–Enzyme Inhibitor) to Determine Impact on Global Mortality and Morbidity in Heart Failure; PLBO, placebo; REPLACE, Replacement of Angiotensin Converting Enzyme Inhibition; RESOLVD, Randomized Evaluation of Strategies for Left Ventricular Dysfunction; SOLVD-prevent, Studies of Left Ventricular Dysfunction–Prevention Trial; SOLVD-treat, Studies of Left Ventricular Dysfunction–Treatment Trial; SPICE, Study of Patients Intolerant of Converting Enzyme Inhibitors; and STRETCH, Symptom, Tolerability, Response to Exercise Trial of Candesartan Cilexetil in Heart Failure.7,21–45
