Abstract
Objectives
Results from the phase 3 Metastatic Pancreatic Adenocarcinoma Clinical Trial (MPACT) led to approval of nab-paclitaxel plus gemcitabine for first-line treatment of metastatic pancreatic cancer. The current analysis evaluated the effects of nab-paclitaxel plus gemcitabine versus gemcitabine on primary pancreatic and metastatic lesions.
Methods
In this analysis of the previously described MPACT trial, changes in pancreatic and metastatic tumor burden were assessed using independently measured diameters of lesions on computed tomography or magnetic resonance imaging scans. Changes in the sums of longest tumor diameters were summarized using descriptive statistics and were included in a multivariate analysis of overall survival.
Results
Primary pancreatic lesion measurement was feasible. Reductions in primary pancreatic tumor burden and metastatic burden from baseline to nadir were significantly greater with nab-paclitaxel plus gemcitabine versus gemcitabine. Baseline pancreatic tumor burden was independently predictive of survival. Both regimens elicited linear reductions in primary pancreatic and metastatic tumor burden through time. There was a high within-patient concordance of tumor changes between primary pancreatic lesions and metastatic lesions.
Conclusions
This analysis of MPACT demonstrated significant tumor shrinkage benefit for nab-paclitaxel plus gemcitabine in both primary pancreatic and metastatic lesions, supporting ongoing evaluation of this regimen in locally advanced disease.
Key Words/Abbreviations: Metastatic Pancreatic Adenocarcinoma Clinical Trial, pancreatic cancer, pancreatic lesions, metastatic, tumor reduction, KPS - Karnofsky performance status, LAPC - locally advanced pancreatic cancer, MPACT - Metastatic Pancreatic Adenocarcinoma Clinical Trial, MPC - metastatic pancreatic cancer, ORR - overall response rate, OS - overall survival, RECIST - Response Evaluation Criteria In Solid Tumors, SLD - sum of longest diameters
Pancreatic cancer is one of the most aggressive tumor types and one of the top 5 causes of cancer-associated death in the United States and European Union.1–4 The overall prognosis for patients with pancreatic cancer remains poor: the 5-year survival rate for all stages of disease combined is only 8%.5 The majority of patients have metastatic disease at the time of diagnosis.6 Approximately 10% to 15% of patients present with resectable localized pancreatic cancer.7 Locally advanced pancreatic cancer (LAPC), which has spread to major blood vessels near the pancreas but not distant sites, is present in up to 25% to 30% of patients at diagnosis.1,6,7
Surgical resection is the only potential cure for pancreatic cancer.4 However, resection is possible in fewer than 20% of patients.8 In the case of borderline resectable pancreatic cancer, neoadjuvant therapy may increase the probability of tumor-free resection margins (R0 resection).8 The primary goal of treating unresectable LAPC is to achieve tumor control. Advances in neoadjuvant chemotherapy and/or chemoradiation have recently improved the possibility of achieving resection.4 However, the benefit of radiation in LAPC remains controversial. For example, the phase 3 LAP 07 trial indicated that gemcitabine-based chemoradiation after induction chemotherapy with gemcitabine did not significantly improve overall survival (OS) compared with gemcitabine-based chemotherapy.9,10 Thus, no single available treatment has demonstrated superiority, and the optimal treatment for borderline resectable or unresectable LAPC remains unknown.
The main goals of treating metastatic pancreatic cancer (MPC) are to palliate symptoms and prolong survival. More than a decade after the first phase 3 advanced pancreatic cancer trial that demonstrated a clinical benefit for gemcitabine, the combination chemotherapy regimen consisting of leucovorin, 5-fluorouracil, irinotecan, and oxaliplatin (FOLFIRINOX) demonstrated greater efficacy and more toxicity versus gemcitabine in patients with MPC in a phase 3 trial.11,12 Recent data from the phase 3 Metastatic Pancreatic Adenocarcinoma Clinical Trial (MPACT) demonstrated that nab-paclitaxel (Abraxane; Celgene Corporation, Summit, NJ) plus gemcitabine had manageable toxicity and superior efficacy versus single-agent gemcitabine as first-line treatment for patients with MPC.13,14 nab-Paclitaxel is an albumin-bound nanoparticle formulation of paclitaxel.15 The combination of nab-paclitaxel and gemcitabine significantly improved OS (median, 8.7 vs 6.6 months; hazard ratio [HR], 0.72; P < 0.001) and promoted long-term survival in a subset of patients with MPC (4% survived ≥36 months).14 Median progression-free survival was also significantly longer in the combination arm (5.5 vs 3.7 months; HR, 0.69; P < 0.001).13 Significant advantages were reported for nab-paclitaxel plus gemcitabine versus gemcitabine alone in terms of overall response rate (ORR) based on the Response Evaluation Criteria In Solid Tumors (RECIST) by independent (23% vs 7%; P < 0.001) and investigator review (29% vs 8%; P < 0.001).13 The most common grade 3 or higher adverse events in the combination arm included neutropenia, leukopenia, fatigue, and peripheral neuropathy. Previous analyses of MPACT demonstrated that Karnofsky performance status (KPS), presence of liver metastases, and age were significantly associated with survival outcomes.14,16 Among patients treated to disease progression in MPACT, treatment exposure, dose intensity, and OS were significantly higher in the nab-paclitaxel plus gemcitabine versus gemcitabine-alone arm (median OS, 9.8 vs 7.5 months; HR, 0.69; P < 0.001).17 In addition, patients in the combination treatment arm demonstrated better preservation of KPS regardless of baseline KPS.18
The positive results of the MPACT trial resulted in the approval of nab-paclitaxel plus gemcitabine as a first-line treatment for MPC.15 However, the effectiveness of this regimen against localized pancreatic cancer remains unknown. The objectives of the current study were to (1) evaluate if primary pancreatic lesions could be measured reliably by independent radiological reviewers and (2) compare responses in primary lesions with those in metastatic lesions of patients in the nab-paclitaxel plus gemcitabine versus gemcitabine monotherapy arm. The second objective is important for understanding the potential of this treatment option in patients with localized pancreatic cancer.
MATERIALS AND METHODS
The study design of the MPACT trial has been described previously.13 Key details are described as follows.
Patients
Adults with KPS 70 or greater and histologically or cytologically confirmed stage IV metastatic pancreatic adenocarcinoma measurable by RECIST version 1.0 were enrolled. Exclusion criteria included prior treatment with chemotherapy for metastatic disease or prior adjuvant systemic chemotherapy, including gemcitabine.
Study Design and Treatment
Patients were randomized 1:1 to receive intravenous nab-paclitaxel 125 mg/m2 followed by intravenous gemcitabine 1000 mg/m2 on days 1, 8, 15, 29, 36, and 43 or intravenous gemcitabine monotherapy 1000 mg/m2 weekly for 7 of 8 weeks. Subsequent cycles for each regimen consisted of treatment on days 1, 8, and 15 of a 28-day cycle. Randomization was stratified by geographic region, performance status, and presence of liver metastases. Patients were treated until disease progression.
Assessments
Spiral computed tomography (CT) or magnetic resonance imaging (MRI) scans were performed every 8 weeks. Lesion size and ORR were evaluated by 2 independent radiological reviewers and an adjudicator according to RECIST version 1.0. Carbohydrate antigen 19–9 levels were evaluated at baseline and every 8 weeks. The sums of the longest diameters (SLDs) for the primary pancreatic and metastatic lesions were calculated to assess changes in tumor burden relative to baseline. Baseline lesion frequency included target and nontarget lesions. Tumor shrinkage measurements were based on SLDs of target lesions. Primary pancreatic lesions referred to lesions found in the head, neck, body, uncinate process, and/or tail of the pancreas, including peripancreatic lymph nodes, both target and nontarget. Target lesions were measurable by RECIST, whereas nontarget lesions were those that were noted but for which measurements were not obtained. Patients with scans of measurable lesions at baseline and 1 or greater postbaseline time point using the same modality (CT or MRI) were included in the analysis.
Statistics
The SLDs at baseline and the percentage change at nadir from baseline were summarized by independent review for pancreatic and metastatic lesions in each treatment arm using descriptive statistics. A nonparametric method, the Wilcoxon rank sum test, was used to test the treatment effect between arms. A multivariate analysis for OS was performed with a proportional hazards model (Cox regression model) for patients without missing values for any variable. The significance level required for entry into the model was 0.20 and for stay was 0.10. The model tested treatment group, geographic region (North America vs others), age, KPS, presence of liver metastases, and baseline pancreatic SLD. Baseline pancreatic SLD was a continuous variable representing the baseline sum of longest diameters of pancreatic lesions. Pearson correlation coefficients were calculated to measure the strength of the linear relationship between the percentage change of pancreatic versus metastatic SLDs from baseline at weeks 8, 16, and 24 for each treatment arm. All statistical tests were 2-sided and performed with SAS versions 9.1 and 9.2 (SAS Institute, Cary, NC). All P values were derived from Wald χ2 tests. The HRs were computed using the exponential function applied to the regression estimate.
RESULTS
Overall Changes in Tumor Burden
This study randomized 861 patients, and this analysis included 652 with evaluable primary pancreatic target lesions and 654 with evaluable metastatic lesions. Primary pancreatic target lesions were reliably measurable with spiral CT or MRI, which allowed for greater precision when evaluating responses. The baseline SLDs of primary pancreatic and metastatic target lesions were similar in the nab-paclitaxel plus gemcitabine and gemcitabine-alone groups (Table 1). The tumor burden was high, with a mean primary pancreatic tumor SLD of approximately 5.0 cm and mean metastatic SLD of approximately 8.5 cm. Primary pancreatic tumor burden and metastatic tumor burden were reduced in both groups after treatment, as indicated by the median and mean percentage reductions in sums of tumor diameters relative to baseline (Table 1). The tumor reductions from baseline to nadir were significantly greater in the nab-paclitaxel plus gemcitabine arm versus gemcitabine-alone arm for both pancreatic (Fig. 1A) and metastatic target lesions (Fig. 1B, Table 1). Overall, there was an approximate threefold greater median percentage reduction in the sum of tumor diameters in the pancreatic and metastatic target lesions of the combination versus gemcitabine arm. Independent review demonstrated that reductions in SLDs for the primary pancreatic target lesions were similar to those of the metastatic target lesions (Table 1). Among patients who had baseline and postbaseline pancreatic lesion measurements, a higher number in the combination arm (127/334 [38%]) demonstrated 30% or greater reduction in SLD from baseline to nadir versus those in the monotherapy arm (48/318 [15%]).
TABLE 1.
Changes in Sums of Tumor Diameters for Primary Pancreatic and Metastatic Target Lesions According to Treatment Arm
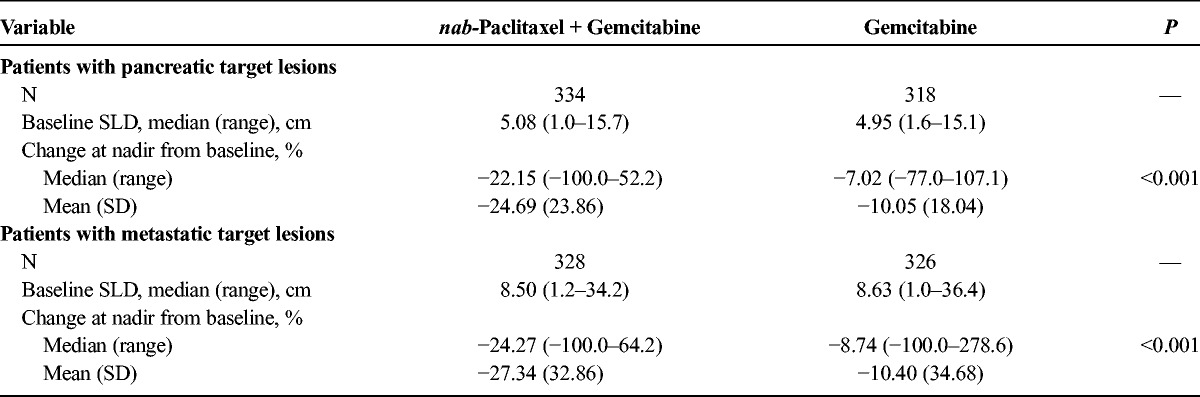
FIGURE 1.
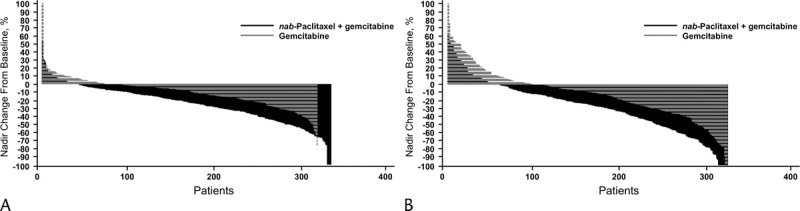
Percentage change in tumor burden from baseline to nadir. The percent change in the sum of the longest diameters of the primary pancreatic (A) or metastatic (B) target lesions was calculated from baseline to nadir in the evaluable population for the gemcitabine monotherapy and nab-paclitaxel plus gemcitabine groups.
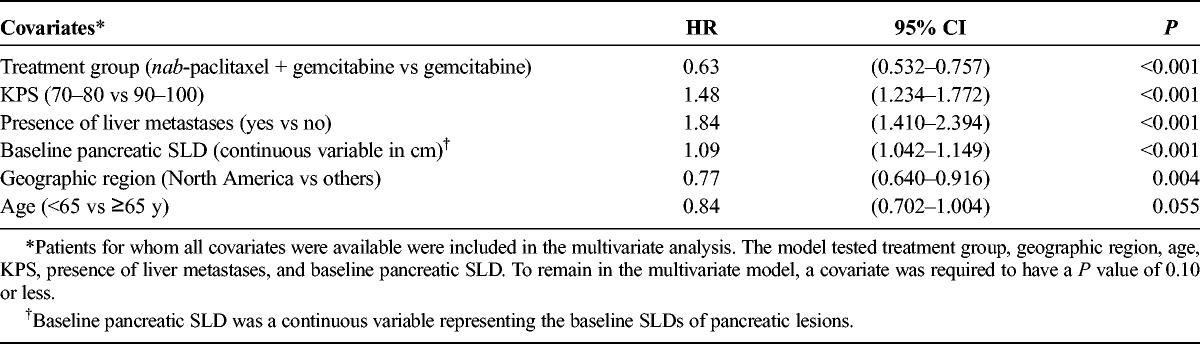
A multivariate analysis (n = 652) was performed to determine the effect of baseline primary pancreatic target lesion SLD on survival (Table 2). The final model included treatment, KPS, presence of liver metastases, geographic region, and age, consistent with previous results.16 Each factor was significantly associated with OS except for age, which did not reach statistical significance (P = 0.055). In addition, baseline pancreatic SLD was found to be an independent predictor of survival (treated as a continuous variable; HR, 1.09; 95% confidence interval [CI], 1.042–1.149; P < 0.001).
TABLE 2.
Multivariate Analysis of OS (n = 652)
The changes in primary pancreatic SLDs from baseline to weeks 8, 16, and 24 were analyzed in each treatment group (Fig. 2). Greater reductions in primary pancreatic target lesion SLDs were observed in the nab-paclitaxel plus gemcitabine versus single-agent gemcitabine arm at all 3 time points. In addition, both treatments seemed to elicit a linear reduction in tumor burden through time. The difference between the nab-paclitaxel plus gemcitabine versus gemcitabine-alone arm was more pronounced within primary pancreatic target lesions (Fig. 2) than in metastatic lesions (Fig. 3), especially at weeks 16 and 24.
FIGURE 2.
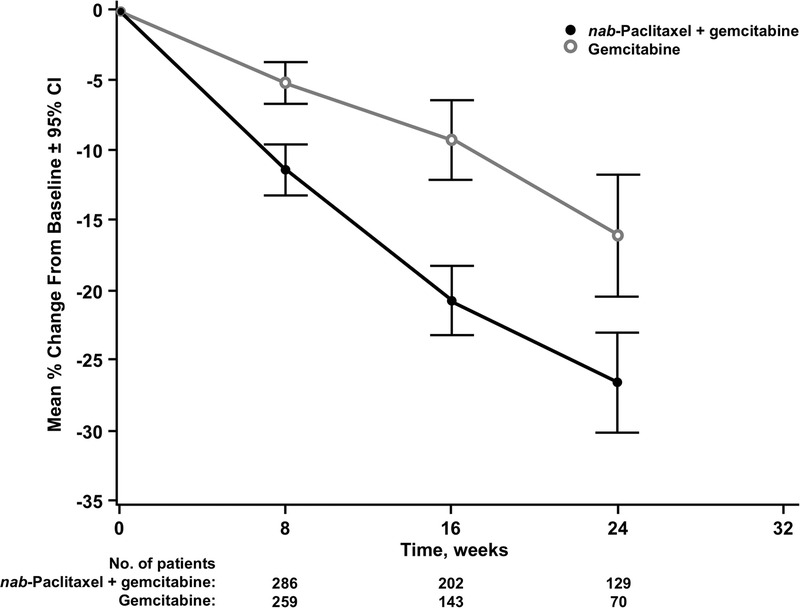
Percentage change in pancreatic target lesion sum of the longest diameters from baseline. The mean percent change in the sum of the longest diameters of the primary pancreatic target lesions in the evaluable population was calculated (±95% CI) from baseline to weeks 8, 16, and 24 for the gemcitabine monotherapy and nab-paclitaxel plus gemcitabine groups.
FIGURE 3.
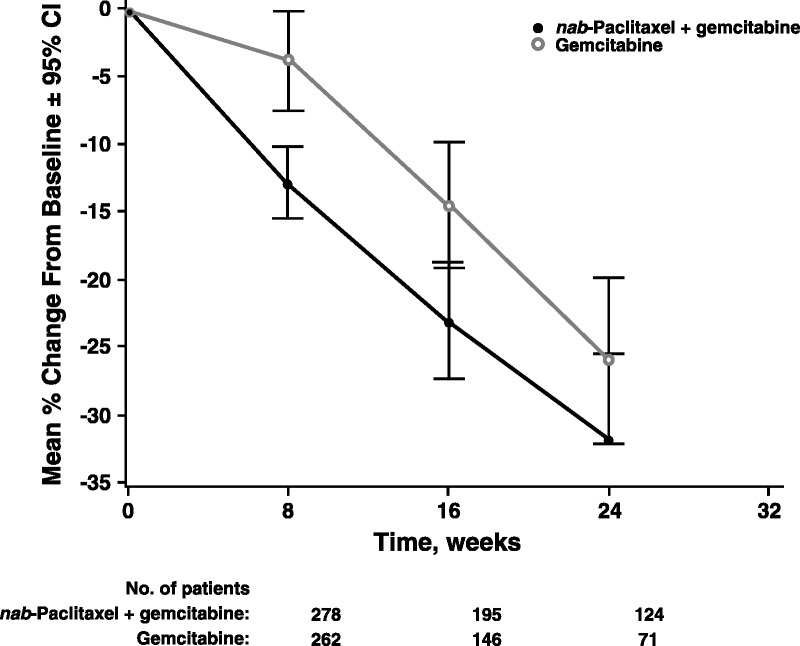
Percentage change in sum of the longest diameters of metastatic target lesions from baseline. The mean percent change in the sum of the longest diameters of the metastatic pancreatic tumors in the evaluable population was calculated (±95% CI) from baseline to weeks 8, 16, and 24 for the gemcitabine monotherapy and nab-paclitaxel plus gemcitabine groups.
Interaction Between Baseline Primary Pancreatic Tumor SLD and Tumor Shrinkage
The correlations between baseline primary pancreatic tumor SLD and changes in primary pancreatic SLD during treatment were examined in each study arm. Patients in the combination arm demonstrated significantly greater tumor shrinkage than those in the gemcitabine-alone arm regardless of baseline tumor SLD. The greatest reduction occurred in patients treated with nab-paclitaxel plus gemcitabine who had primary pancreatic SLDs less than 5.0 cm at baseline, which was a decrease more than threefold greater than that for gemcitabine alone (−28.4% vs −8.7%; P < 0.001). The combination also demonstrated significantly greater tumor shrinkage (almost 2-fold) than gemcitabine alone in tumors 5.0 cm or greater (−21.3% vs −11.5%; P < 0.001). Within treatment arms, only the combination demonstrated significant differences between reductions in tumor burden for patients with SLDs that were less than 5.0 cm versus 5.0 cm or greater at baseline (−28.4% vs −21.3%; P = 0.012). In the gemcitabine-alone arm, tumor shrinkage was not significantly different between larger versus smaller SLDs at baseline (−11.5% vs −8.7%; P = 0.621).
Correlation of Primary and Metastatic Lesion Changes Within Patients
To determine if primary pancreatic and metastatic target lesions demonstrated the same dynamics within a given patient, percentage changes in primary pancreatic target lesion SLDs and metastatic target lesion SLDs were calculated for each patient at weeks 8, 16, and 24 of treatment. Patients in the nab-paclitaxel plus gemcitabine arm showed positive, statistically significant correlations between changes in primary and metastatic target lesions at each time point (Fig. 4). In the gemcitabine-alone arm, there were significant correlations between changes in primary and metastatic lesions at weeks 8 and 16 but not at week 24.
FIGURE 4.
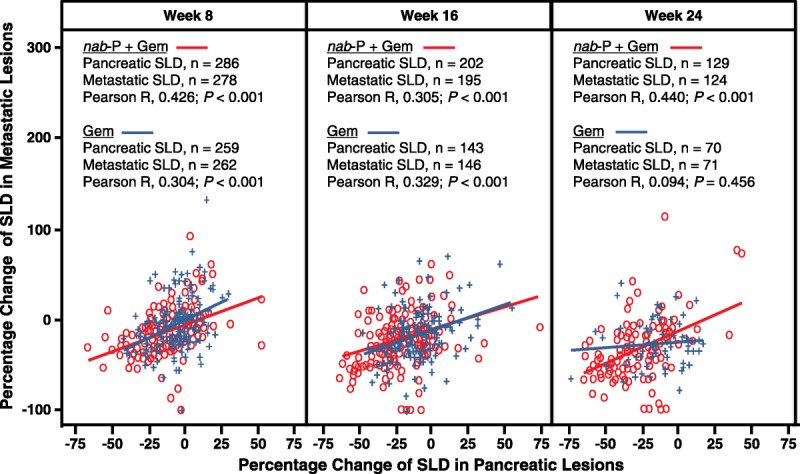
Within-patient correlation between percentage change in pancreatic target lesion burden and metastatic target lesion burden. Distribution of percent change in metastatic burden from baseline versus percent change in pancreatic tumor burden from baseline is shown at weeks 8, 16, and 24 of treatment for each patient in the combination arm (open red circles) and each patient in the gemcitabine-alone arm (blue crosses). Linear fits were performed for data points in the combination group (solid red line) and gemcitabine-alone group (solid blue line). Gem, gemcitabine; nab-P, nab-paclitaxel.
CONCLUSIONS
This analysis is the first to evaluate the effects of treatment on primary pancreatic and metastatic lesions within the same patient population in a large, prospective global phase 3 study. Primary lesions were reliably measured with spiral CT or MRI. Patients had a high pancreatic tumor burden with large baseline tumor SLDs (median, approximately 5.0 cm) and an almost 70% larger sum of metastatic target lesions (median, approximately 8.5 cm). Spiral CT also allowed changes in primary and metastatic SLDs to be assessed through time. Primary and metastatic lesions demonstrated similar patterns of tumor shrinkage. Significantly greater reductions in tumor burden at primary pancreatic and metastatic sites were seen in patients treated with the combination of nab-paclitaxel plus gemcitabine compared with gemcitabine alone. These results are consistent with the superior ORR demonstrated for nab-paclitaxel plus gemcitabine versus gemcitabine alone.13 Furthermore, greater reductions in primary pancreatic burden were observed for the combination arm at 8, 16, and 24 weeks. Importantly, this new analysis demonstrated that the primary pancreatic burden was reduced to a similar extent as the metastatic burden both within patients and within each study arm.
The majority of patients with LAPC eventually develop distant metastases, with data supporting a genetic progression from LAPC to MPC.19 Thus, understanding both the primary pancreatic and metastatic tumor dynamics after treatment with nab-paclitaxel plus gemcitabine is relevant to localized pancreatic cancer, especially LAPC. The reductions in primary pancreatic tumor burden observed in this study suggest that nab-paclitaxel plus gemcitabine may be an effective chemotherapeutic option for LAPC.
In the nab-paclitaxel plus gemcitabine treatment arm, there was a greater percentage shrinkage in smaller versus larger pancreatic tumor burden (SLD < 5.0 cm vs ≥5.0 cm). Despite the potential impact of tumor size on treatment efficacy, analysis of interactions between baseline tumor size and tumor burden change showed that nab-paclitaxel plus gemcitabine resulted in significantly greater shrinkage of even the largest pancreatic tumors compared with gemcitabine alone. Both treatment arms exhibited a linear reduction in primary pancreatic and metastatic tumor burden until week 24. However, the slope of the reduction was much steeper in the combination group (ie, tumors of patients in the combination arm demonstrated greater and faster shrinkage versus tumors treated with gemcitabine alone).
The stromal makeup of pancreatic tumors is thought to be a critical factor contributing to the degree of therapeutic response.20 Potential similarities in the stroma surrounding primary and metastatic lesions may in part explain similarities in the extent to which primary and metastatic burden is reduced.21 Because a majority of patients present with late-stage cancer, the finding that nab-paclitaxel plus gemcitabine remains effective in larger tumors further supports the use of this combination in advanced disease. This finding may be particularly applicable to the treatment of LAPC, especially conversion of unresectable tumors to resectable and potentially curable disease.
Treatment of LAPC remains highly controversial. Optimal treatment strategies need to be verified in prospective clinical trials. Nevertheless, recent identification of more active systemic chemotherapy regimens, such as nab-paclitaxel plus gemcitabine, has engendered increased interest in neoadjuvant approaches for patients with LAPC. These newer chemotherapy combinations also provoke questions regarding the benefit of radiation therapy in LAPC, particularly because a recent phase 3 trial comparing chemoradiation with gemcitabine-based chemotherapy failed to demonstrate an improvement in OS in this setting.7 Thus, clinical trials that help clarify which therapies reduce primary pancreatic and metastatic tumor burdens will allow more effective treatments to be identified for LAPC.
The results presented here suggest that nab-paclitaxel plus gemcitabine effectively reduced primary pancreatic tumor burden even in cases with relatively large tumor burden. These data provide rationale for conducting prospective trials testing nab-paclitaxel plus gemcitabine in patients with LAPC. The value of nab-paclitaxel plus gemcitabine-based neoadjuvant chemotherapy without chemoradiation in LAPC will be addressed in 2 ongoing prospective trials. The locally advanced pancreatic adenocarcinoma clinical trial, an ongoing international, nonrandomized, open-label, multicenter phase 2 study, is evaluating nab-paclitaxel plus gemcitabine in patients with unresectable, previously untreated LAPC.22 In addition, a prospective, randomized, multicenter phase 2 trial, the neoadjuvant chemotherapy in locally advanced pancreatic cancer trial, will test the efficacy and safety of nab-paclitaxel plus gemcitabine for 2 cycles followed by either 2 additional cycles of nab-paclitaxel plus gemcitabine or additional cycles of 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin as neoadjuvant chemotherapy in borderline and unresectable LAPC.23 An exploratory end point of both trials is to evaluate biomarkers for the potential to predict tumor response to nab-paclitaxel plus gemcitabine.
In summary, this analysis of the MPACT study supports the significant benefit of nab-paclitaxel plus gemcitabine against primary pancreatic and metastatic lesions. Significant decreases in the size of primary pancreatic tumors suggest that nab-paclitaxel plus gemcitabine may be an effective treatment approach for patients with LAPC, a concept that will be evaluated in ongoing and future clinical trials.
ACKNOWLEDGMENT
The authors thank Rita Nahta, PhD, MediTech Media, Ltd, for providing writing assistance.
Footnotes
This study was supported by Celgene Corporation.
V.K. has a consultant/advisory role and receives research funding from Celgene. R.R. has a consultant/advisory role and receives honoraria and research funding from Celgene. D.G. has a consultant/advisory role and receives research funding from Celgene. H.L., S.F., B.L., and M.R. are employees of and have stock ownership in Celgene. D.V.F. has a consultant/advisory role and receives honoraria from Celgene and receives research funding from Honor Health.
The authors were fully responsible for all content and editorial decisions for this article.
REFERENCES
- 1.National Cancer Institute-Surveillance, Epidemiology, and End Results Program. SEER stat fact sheets: pancreas cancer. Available at: http://seer.cancer.gov/statfacts/html/pancreas.html. Accessed June 17, 2016.
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 3.Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v56–v68. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. Clinical practice guidelines in oncology. Pancreatic adenocarcinoma. V1. 2016. Available at: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed June 17, 2016.
- 5.American Cancer Society. Cancer facts and figures. 2016. Available at: http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed June 17, 2016.
- 6.Heinemann V, Haas M, Boeck S. Neoadjuvant treatment of borderline resectable and non-resectable pancreatic cancer. Ann Oncol. 2013;24:2484–2492. [DOI] [PubMed] [Google Scholar]
- 7.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:2140–2141. [DOI] [PubMed] [Google Scholar]
- 8.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. [DOI] [PubMed] [Google Scholar]
- 9.Hammel P, Huguet F, Van Laethem JL, et al. Comparison of chemoradiotherapy (CRT) and chemotherapy (CT) in patients with a locally advanced pancreatic cancer (LAPC) controlled after 4 months of gemcitabine with or without erlotinib: final results of the international phase III LAP 07 study. J Clin Oncol. 2013;31(suppl):[abstract LBA4003]. [Google Scholar]
- 10.Huguet F, Hammel P, Vernerey D, et al. Impact of chemoradiotherapy (CRT) on local control and time without treatment in patients with locally advanced pancreatic cancer (LAPC) included in the international phase III LAP 07 study. J Clin Oncol. 2014;32(suppl):4001. [Google Scholar]
- 11.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. [DOI] [PubMed] [Google Scholar]
- 12.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. [DOI] [PubMed] [Google Scholar]
- 13.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein D, El-Maraghi RH, Hammel P, et al. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst. 2015;107:dju413. [DOI] [PubMed] [Google Scholar]
- 15.Celgene Corporation. Abraxane for Injectable Suspension (Paclitaxel Protein-Bound Particles for Injectable Suspension; Albumin-Bound). Summit, NJ: Celgene Corporation; 2014. [Google Scholar]
- 16.Tabernero J, Chiorean EG, Infante JR, et al. Prognostic factors of survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Oncologist. 2015;20:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vogel A, Penenberg D, McGovern D, et al. Efficacy and safety of nab-paclitaxel (nab-P) plus gemcitabine (gem) vs gem alone in patients (pts) with metastatic pancreatic cancer (MPC) treated to progressive disease (PD) in the phase III MPACT trial. Eur J Cancer. 2015;51(suppl 3):S455. [Google Scholar]
- 18.Chiorean EG, Wan Y, Whiting S, et al. Impact of nab-paclitaxel (nab-P) plus gemcitabine (G) vs gemcitabine alone on Karnofsky performance status (KPS) in metastatic pancreatic cancer pts with good or poor performance status at baseline: a post-hoc analysis of the MPACT trial. Eur J Cancer. 2015;51(suppl 3):S450. [Google Scholar]
- 19.Abramson MA, Jazag A, van der Zee JA, et al. The molecular biology of pancreatic cancer. Gastrointest Cancer Res. 2007;1(4 suppl 2):S7–S12. [PMC free article] [PubMed] [Google Scholar]
- 20.Whatcott C, Han H, Posner RG, et al. Tumor-stromal interactions in pancreatic cancer. Crit Rev Oncog. 2013;18:135–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whatcott CJ, Diep CH, Jiang P, et al. Desmoplasia in primary tumors and metastatic lesions of pancreatic cancer. Clin Cancer Res. 2015;21:3561–3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Philip PA, Lacy J, Dowden SD, et al. LAPACT: An open-label, multicenter phase II trial of nab-paclitaxel (nab-P) plus gemcitabine (Gem) in patients with locally advanced pancreatic cancer (LAPC). J Clin Oncol. 2016;34(suppl):TPS477. [Google Scholar]
- 23.ClinicalTrials.gov. Trial to investigate intensified neoadjuvant chemotherapy in locally advanced pancreatic cancer (NEOLAP). Available at: https://www.clinicaltrials.gov/ct2/show/NCT02125136. Accessed February 3, 2016.


