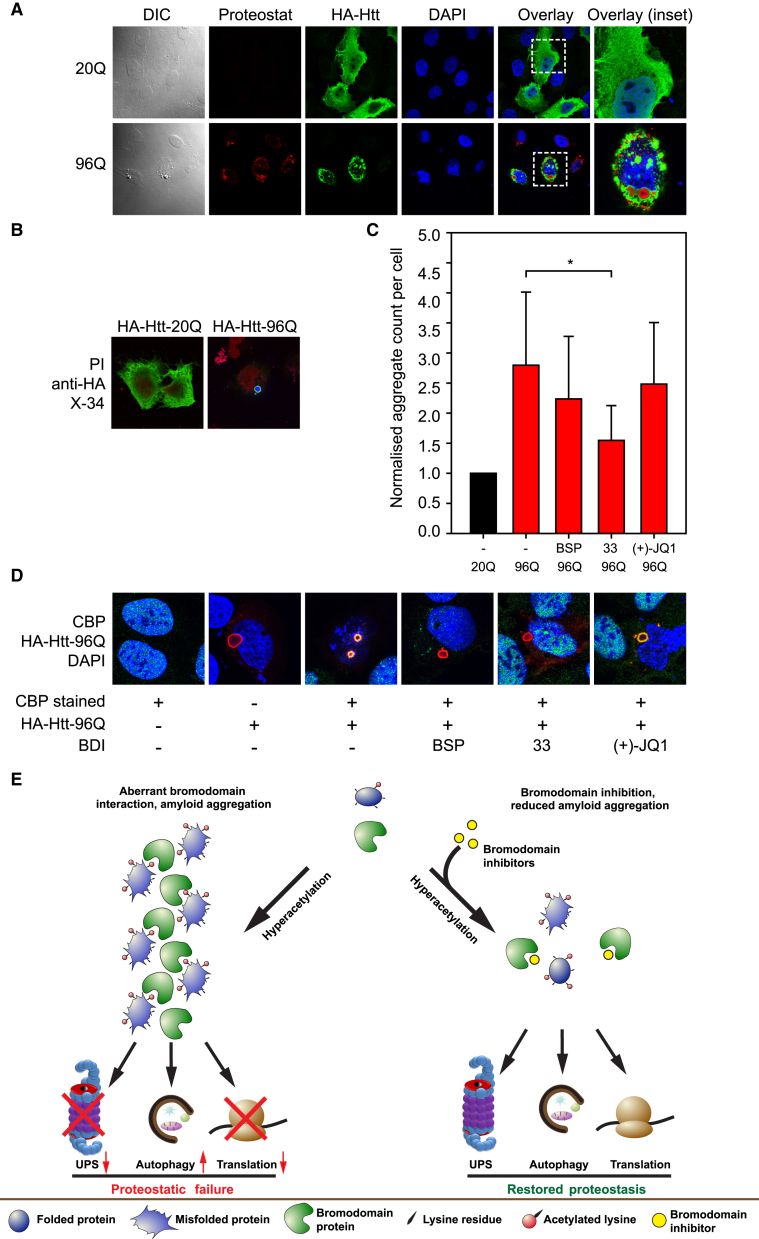
Figure 7.
Effect of BDIs on Pathologically Elongated Huntingtin
(A) U2OS cells expressing either the exon 1 of huntingtin (Htt) with 20Q or 96Q were fixed, permeabilized, and stained with an HA antibody (shown in green). Proteostat was applied to visualize aggregated structures (red), and nuclei were counterstained with DAPI (blue). Panels on the right represent the enlarged area shown by a dashed square on panels to the left.
(B) U2OS cells expressing HA-Htt-96Q exon 1 form aggregates and are positively stained with X-34 (shown in blue). Nuclei were stained with propidium iodide (PI) (red) and the Htt constructs are shown in green.
(C) U2OS cells expressing HA-Htt-96Q or HA-Htt-20Q were treated with the respective BDI (2.5 μM), then fixed, permeabilized, and stained with Proteostat and DAPI as a nuclear counterstain. The number of Proteostat-positive aggregates was then quantified with an IN Cell Analyzer 1000. The data were derived from at least three independent experiments each in triplicates, and for each condition at least 1,500 cells were analyzed relative to 20Q, which was given an arbitrary value of 1. Level of statistical significance is indicated (*p ≤ 0.05). Error bars denote SD. The sample expressing HA-Htt-20Q is depicted in black, and HA-Htt-96Q-expressing cells are depicted in red. BSP, bromosporine; 33, compound 33; (+)-J, (+)-JQ1.
(D) U2OS cells expressing HA-Htt-96Q were treated with either DMSO (−) or BDIs (2.5 μM), then fixed, permeabilized, and stained with an antibody against CBP (green), HA (red), and DAPI (blue) as a nuclear counterstain. Co-localization of HA-Htt-96Q aggregates with endogenous CBP is depicted by yellow/orange color. Shown are the overlays from the three different channels. BSP, bromosporine; 33, compound 33; (+)-J, (+)-JQ1.
(E) Schematic overview of protein aggregation and proteostatic failure upon hyperacetylation and rescue by bromodomain inhibitors. Proteins aggregate upon hyperacetylation and aberrant interactions occur with bromodomain-containing proteins such as CBP/p300. Essential protein quality control mechanisms are affected, such as degradation of proteins via the ubiquitin proteasome system and protein translation. BDIs, and especially those preferentially binding to CBP/p300, diminish the formation of aggregates and also restore to some extent protein degradation and translation.
See also Figure S7.