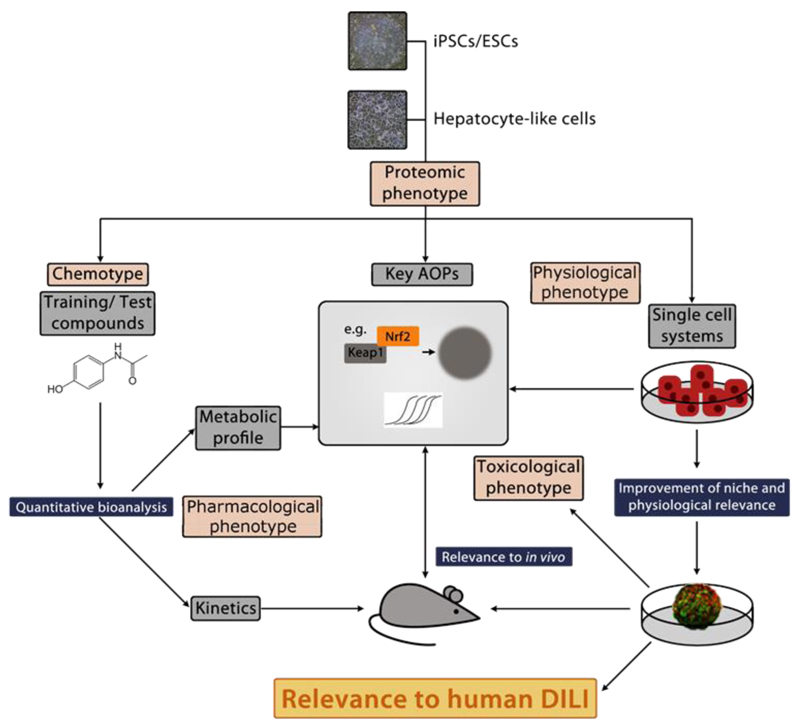
Figure 1. Roadmap for producing stem cell-derived models to improve mechanistic understanding and prediction of human DILI.
The physiological, pharmacological and toxicological characterisation of stem cell-derived hepatocytes is necessary before the cells can be fully utilised. This will include the use of toxicity/stress reporters, and a small panel of well-defined chemicals, thereby defining the toxicological purpose for which each line is suitable. This will position the new cells within a screening toolbox that could be validated for drug/chemical safety evaluation. The use of iPSC lines with drug toxicity-relevant mutations and the use of CRISPR technology to edit genes involved in drug metabolism may also be important in this regard.
Abbreviation: AOP = Adverse Outcome Pathway