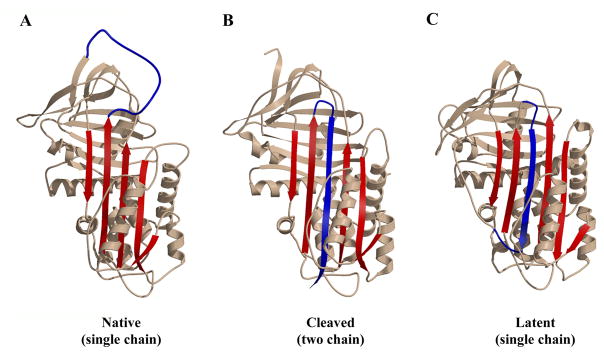
Fig 1. Principal serpin conformations.
The native state (panel A, α1PI pdb code 1QLP [176]) and latent state (panel C, PAI-1 pdb code 1CG5 [177]) represent the metastable and most stable states respectively of the single-chain forms of the serpin. They differ principally in the location of the RCL (blue) as being exposed (native) or integrated into β-sheet A (latent) and of s1C as being part of β-sheet C (native) or an unstructured exposed linker (latent). Cleavage of the RCL permits the serpin to have both the RCL integrated into β-sheet A and s1C still as part of β-sheet C (panel B, α1PI pdb code 7API [178]). The remainder of β-sheet A is shown in red. β-sheet C is top left and β-sheet B is behind β-sheet C. Most helices pack on the “back” face of the serpin in this presentation, with the major exceptions of helix F (across the front of β-sheet A) and helices D and E (D above E) on the right edge. Adapted from ref [179]. Copyright Elsevier.