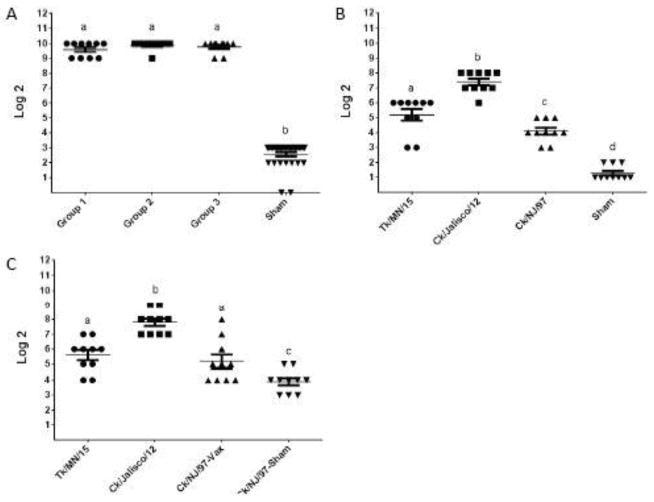
Figure 3.
Immunogenicity of H5/H7/H9/N1/gag VLPs. X-axis indicates Log2 dilution of sera, y-axis indicated antigen tested. (A) Pre-challenge serum antibody, by HI assay using VLP as antigen. Groups 1, 2, and 3 were vaccinated with VLPs and later challenged with H5, H7 and H9 viruses, respectively. For vaccinations, VLPs were formulated with SEPPIC adjuvant to contain 1,536 HA units per dose (512 HA units of each subtype) and administered to chickens at day 1 and day 21 of age. (B) Pre-challenge serum antibody, by HI assay using indicated virus antigen prepared from A/turkey/Minnesota/7172-1/2015 (H5N2), A/chicken/Jalisco/CPA1/2012 (H7N3) and A/chicken/New Jersey/12220/1997 (H9N2) viruses. (C) Post-challenge serum antibody, by HI assay using indicated virus antigens.