Abstract
Two diffusion-based approaches, CG (constant gradient) and FEXI (filtered exchange imaging) methods, have been previously proposed for measuring transcytolemmal water exchange rate constant kin, but their accuracy and feasibility have not been comprehensively evaluated and compared. In this work, both computer simulations and cell experiments in vitro were performed to evaluate these two methods. Simulations were done with different cell diameters (5, 10, 20 µm), a broad range of kin values (0.02 – 30 s−1) and different SNR’s, and simulated kin’s were directly compared with the ground truth values. Human leukemia K562 cells were cultured and treated with saponin to selectively change cell transmembrane permeability. The agreement between measured kin’s of both methods was also evaluated. The results suggest that, without noise, the CG method provides reasonably accurate estimation of kin especially when it is smaller than 10 s−1, which is in the typical physiological range of many biological tissues. However, although the FEXI method overestimates kin even with corrections for the effects of extracellular water fraction, it provides reasonable estimates with practical SNR’s and more importantly, the fitted apparent exchange rate AXR showed approximately linear dependence on the ground truth kin. In conclusion, either CG or FEXI method provides a sensitive means to characterize the variations in transcytolemmal water exchange rate constant kin, although the accuracy and specificity is usually compromised. The non-imaging CG method provides more accurate estimation of kin, but limited to large volume-of-interest. Although the accuracy of FEXI is compromised with extracellular volume fraction, it is capable of spatially mapping kin in practice.
Keywords: diffusion, MRI, transcytolemmal water exchange, membrane permeability, constant gradient, filter exchange imaging, CG, FEXI, saponin
Graphical abstract
Both diffusion-based CG (constant gradient) and FEXI (filtered exchange imaging) methods were evaluated and compared for measuring transcytolemmal water exchange rate constant kin using computer simulations and cell experiments in vitro. Although compromised with accuracy and specificity, the FEXI method provides a sensitive way to measure kin with more clinical potential.
INTRODUCTION
Transcytolemmal water exchange across cellular membranes plays an important role in a large variety of pathophysiological processes of living cells [1, 2]. It has been found that cell transmembrane permeability is correlated with cell membrane fluidity, which is an important indicator of living cell status [2]. In addition to these non-equilibrium water exchange, the equilibrium cell transmembrane permeability is also important and has been found to be increased significantly in tumors [3, 4] as well as neurodegenerative diseases such as Parkinson’s disease [5], and Alzheimer disease [6]. Therefore, the measurement of transmembrane permeability may provide insights for more specific assessments of the pathophysiological status of biological tissues.
In traditional biology, the cell transmembrane permeability or transcytolemmal water exchange is typically measured in cultured cells and the usage of such a method is limited in clinical practice [7]. By contrast, MRI provides a powerful and unique means capable of non-invasively mapping overall averaged transcytolemmal water exchange in vivo, although it is incapable of distinguishing permeabilities due to facilitated diffusion or osmosis. The current MRI methods measuring transcytolemmal water change can be summarized into two categories, i.e. relaxation- and diffusion-based methods. Both methods usually assume a two-component system (i.e. intra- and extra-cellular spaces) with water exchange at a single averaged rate constant (i.e. kin, the transcytolemmal water exchange rate constant from intra- to extracellular space, whose relation to the cell transmembrane permeability Pm is given below in Eq.(1)). Relaxation-based methods usually use Gd-based contrast agents to selectively alter extracellular relaxation properties, and hence provide an opportunity to investigate the water exchange between intra- and extra-cellular spaces [3]. One disadvantage of this method is that it relies on the delivery of contrast agents to the extracellular space, which may be significantly limited in practice such as in brain regions with an intact blood-brain barrier (BBB). Moreover, multiple injections of Gd-based agents may increase the risk of nephrogenic systemic fibrosis in patients with concurrent acute or chronic kidney diseases [8].
By contrast, the diffusion-based methods do not require any exogenous probes and hence provide a more flexible and safer means to quantify transcytolemmal water exchange without such limitations. The behavior of two-component systems in diffusion measurements was thoroughly investigated by Karger et al. by assuming two freely-diffusing fractions [9], and later was improved to include restrictions on the intracellular water compartment [10–12]. Specifically, the CG (constant diffusion gradient) method has been developed to measure kin by keeping diffusion gradients constant while varying diffusion times [13, 14]. However, the demand of very high b values (e.g. 580 ms/µm2 in rat brain in vivo [13]) in the CG method constrains its practical applications, and hence it has been implemented only to measure the averaged kin of a large volume-of-interest in a live rat brain in vivo in a non-imaging (spectroscopic) manner [13]. Recently, a filtered-exchange imaging (FEXI) method has been developed to measure apparent water exchange rate constants (AXR) [15–18]. Such a method employs a diffusion filter block and a diffusion detection block separated by a mixing time tm. Although the obtainable apparent exchange rate AXR is actually kin divided by the extracellular water fraction fex, the FEXI method provides a sensitivity to map kin in humans in vivo [4, 16, 18, 19]. The FEXI method has been used to characterize meningioma and astrocytoma brain tumors [4, 18] and breast tumors [16], suggesting its clinical potential. However, despite its successful implementation, the FEXI method has not been fully validated before. Heating [15] and detergent [17] treatments have been performed to change cell membrane permeability in vitro in order to investigate the sensitivity of AXR to kin, but these manipulations can also cause concurrent variations in other properties e.g. changes in intracellular diffusivity and cell size, which complicate data interpretation. Moreover, computer simulations, a powerful tool to investigate the direct relationship between microstructure and MR signals, have not yet been carried out to evaluate the accuracy of AXR obtained using the FEXI method.
In the current study, we use both computer simulations and well-controlled cell cultures in vitro with varying cell membrane permeabilities selectively to evaluate and compare the CG and FEXI methods. In computer simulations, the exchange rate constants obtained from both diffusion methods were compared directly with ground-truth values so that the accuracies of both methods could be assessed. In cell studies in vitro, saponin was used in fixed cells to selectively change overall cell membrane permeability without altering other cellular microstructures [20], which provides a means to precisely investigate how cell transmembrane permeability influences the measurements of kin in both methods. Limitations and possible applications of both methods in practice are discussed.
MATERIALS AND METHODS
Theory
Both CG and FEXI methods assume a two-component system containing intra- and extracellular spaces. The transcytolemmal water exchange rate constant kin is defined as the reciprocal of the averaged lifetime of intracellular water molecules τin, i.e. kin = 1/ τin. kin indicates the exchange rate constant from intra- to extracellular space. In a 3D system consisting of spherical cells, the cell transmembrane permeability Pm is related to kin as [21, 22]
| (1) |
where d is the spherical cell diameter and Din0 is the intrinsic intracellular diffusivity. Din0 influences the measurements of Pm, as reported previously [23]. Note that, with the physiology relevant values of d, Din, and kin, the first term on the right side of Eq.(1) is typically much larger than the second term so that it usually dominates Pm.
CG (constant gradient) method
The pulse sequence of the CG method is shown in Figure 1A with g is the gradient strength, δ the gradient duration and Δ the gradient separation. With the short pulse approximation, the diffusion time tD ≈ Δ. The diffusion MR signals arising from a two-component system with an average exchange rate constant can be expressed as
| (2) |
where Sin and Sex are signals arising from intra- and extracellular spaces, Din and Dex denote apparent intra- and extracellular diffusivities, kex = finkin / fex is the transcytolemmal water exchange rate constant from the extra- to intracellular space, fin and fex are the water fractions of the intra- and extracellular spaces, respectively, and q̃ = γδg, with γ being the gyromagnetic ratio. The solution to Eq.(2) based on Gaussian diffusion and a single diffusion encoding sequence is
| (3) |
where DA, DB and pA, pB are referred to as apparent diffusion coefficients and apparent volume fractions, respectively, and their specific relation to real system parameters has been reported previously [11, 12]. In constant gradient (CG) measurements, if two conditions are satisfied [24], i.e. 1) a sufficiently long diffusion time is used, i.e. 4·tD ·Din / d2 >> 1, and 2) the transcytolemmal water exchange rate constant is slow, i.e. q̃2Dex >> kin, kin is given by [14]
| (4) |
where DB is the ADC when b = (γδg)2 (Δ − δ / 3) is sufficiently high with a constant gradient g. Note that it has been reported that the applicability of the Karger Model also requires q̃2·lc << 1, where lc is the characteristic diffusion length on the order of cell size [24].
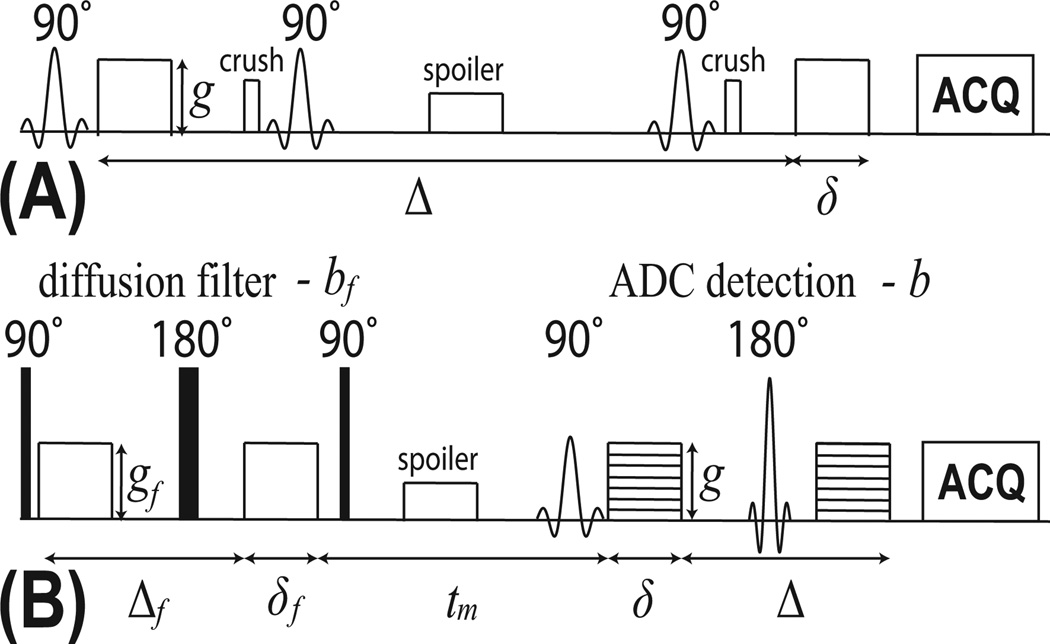
Figure 1.
Pulse sequence diagrams of (A) CG and (B) FEXI methods.
FEXI (filtered exchange imaging) method
Figure 1B demonstrates the sequence of the FEXI method, which consists of a “diffusion filter” followed by a diffusion detection block. The diffusion filter serves as a low-pass diffusion filter that changes the relative signal fractions of the intra- and extra-cellular spaces when they have different intrinsic diffusivities, and the diffusion detection block detects the relative signal contributions [4, 15–17]. The signals from the FEXI method are dependent on the mixing time tm as
| (5) |
where the tm-dependent apparent diffusion coefficient ADC'(tm) is related to the exchange rate as
| (6) |
where σ is the filter efficiency, and the apparent exchange rate AXR is actually kin modulated by the extracellular volume fraction, namely, AXR
| (7) |
Note that the equilibrium condition fin · kin = fex · kex was used to derive Eq. (7). Therefore, unlike the direct measurement of kin in the CG method, an apparent exchange rate AXR can be obtained from the FEXI method, which is proportional to kin and more importantly AXR can be mapped in human subjects in vivo. Note that FEXSY (the spectroscopic version of FEXI) is capable of estimating the exchange rate constants by using large range of b values to separate intra- and extra-cellular contributions, and theoretically provide more accurate estimation of kin not confounded by the extracellular fraction [15]. However, since the CG method has long been implemented in various applications by multiple groups, it is more suitable to represent the non-imaging diffusion methods to measure kin. Therefore, FEXSY will not be discussed in the current work.
Cell sample preparation
Human myelogenous leukemia K562 cells were purchased from American Type Culture Collection (Manassas, Virginia, USA) and prepared as described previously [20]. Briefly, cells were cultured in Dulbecco’s modified eagle medium (DMEM) supplemented with 10% fetal bovine serum, 50 U/mL penicillin, and 50 µg/mL streptomycin (Invitrogen, Carlsbad, California, USA) under standard culture conditions in a humidified incubator maintained at 5% CO2 and 37°C. Once enough cells were cultured, they were fixed and divided into three groups, each treated with vehicle (control) and low (0.025% w/v) and high (0.05% w/v) concentrations of saponin (Sigma-Aldrich, St. Louis, Missouri, United States). The cells were fixed by 4% paraformaldehyde for 2 hours and with saponin solution for 30 mins. Saponin is a type natural detergent that selectively removes membrane cholesterols and increases cell membrane permeability without altering other cell properties [25, 26]. Cells were centrifuged at g = 6000 for 2 mins. There were six pellets in each group, and each pellet contained approximately 30 million cells in a 0.65-mL Eppendorf tube. Note that fixation removes cell vitality which affects the transcytolemmal water exchange. However, the usage of saponin provides a means to increase passive diffusion so that overall averaged transcytolemmal water exchange rate constant can vary such as in physiologically relevant range with appropriate preparation.
MR experiments
All diffusion measurements were performed on an Agilent/Varian 4.7T MRI system with a 38-mm Doty coil for both transmission and reception. The magnet bore temperature was maintained at ~ 17°C using a cooling water circulation system. The non-imaging constant gradient NMR experiments were performed with a stimulated echo (STEAM) diffusion sequence as shown in Figure 1A. A 3-mm axial slice covering the central cell pellets was excited for the collection of NMR signals. The diffusion gradient duration δ = 10 ms, and the gradient separation Δ was varied in 30 increments. The maximum Δ was 832, 368, and 136 ms for cell samples with saponin concentrations of 0, 0.025%, and 0.05%, respectively. Diffusion gradients were applied on three axes simultaneously with amplitude g = 50 mT/m on each axis. To compensate for T1 relaxation effects during the mixing time, the experiment was repeated with a lower gradient amplitude g = 5 mT/m to normalize the higher gradient datasets as described in [13, 20]. Other parameters included: repetition time (TR) = 3.5 sec; echo time (TE) = 25 ms; number of excitations (NEX) = 2; receiver bandwidth = 50 kHz and spectral resolution = 390.625 Hz. The total scan time for each constant gradient experiment was ~ 6 min.
The FEXI experiments were conducted with a diffusion-filtered spin-echo echo-planar imaging (SE-EPI) diffusion sequence as shown in Figure 1B. Diffusion gradients were applied on three axes simultaneously with duration δ = 6 ms in both the filter and detection blocks. The gradient separation Δf = 40 ms in the filter block and Δ = 20 ms in the detection block. The diffusion weighting bf = 3000 s/mm2 in the filter block and b = 0 and 1500 s/mm2 in the detection block. Apparent diffusion coefficients (ADCs) were measured for 19 mixing times tm: 7, 16, 26, 36, 46, 56, 66, 76, 86, 96, 106, 126, 146, 166, 186, 206, 256, 1006, 3006 ms. The equilibrium ADC was acquired with the filter inactive (bf = 0 s/mm2) and tm = 7 ms. Other parameters included: TR = 3.5 sec; TE = 38 ms; field of view = 25 × 25 mm; matrix size = 32 × 32; slice thickness = 3 mm and NEX = 8. The scan time for each mixing time tm was ~ 3 min, yielding a total scan time of 60 min for each FEXI experiment. Note that there were altogether 30 signal data points acquired in the CG measurements and 20 signal data points acquired in the FEXI measurements.
Computer simulations
An improved finite difference method [27] was used in the simulations which incorporates the cell transmembrane permeability as described in [28]. The computer simulations were performed on modeled tissues as a collection of densely packed spherical cells positioned on a three-dimensional face-centered cubic structure (FCC) [29]. Water molecules were allowed to diffuse in intra- and extracellular spaces with a cell membrane of finite permeability separating the compartments. The simulation assumed Dex = 2 µm2/ms, which is the diffusion coefficient of free water at 20°C [30], and Din = 1 µm2/ms, a half of the free water diffusion coefficient [31]. We anticipate Dex was close to the intrinsic diffusion coefficient of cell medium used in the experiments, while Din may have minor effects on simulated results because the diffusion times used were relatively long. Three different cell diameters 5, 10, and 20 µm were simulated while the intracellular volume fraction was fixed as 61.8%. 18 ground truth kin values were simulated in both CG and FEXI simulations: 0.02, 0.05, 0.1, 0.15, 0.3, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 5, 10, 15, 20, 25, 30 s−1, corresponding to a very broad range of intracellular water lifetime τin from 33 ms to 50 sec. Note that intracellular water lifetime τin has been reported as 622±29 ms [13] or ~ 550 ms [32] in normal brain tissues and ~ 1.67 sec in mouse xenografts in vivo [3]. Therefore, the simulated range of intracellular water lifetimes covered the physiological relevant values. 19 mixing times were used in the FEXI simulations: 7, 20, 30, 50, 100, 150, 200, 300, 400, 500, 600, 800, 1000, 1500, 2000, 2500, and 3000 ms. All other parameters were the same as those used in the CG and FEXI measurements. For simplicity, the influences of relaxation were ignored in the simulations. Note that our simulations were based on a simplified tissue model that assumes cells are regularly packed, but recent findings have suggested this may lead a biased estimation of diffusion signals compared with more realistic random packing [33]. The total simulation time was ~14.5 hours for all of the CG simulations and ~14 hours for all the FEXI simulations.
To investigate the influence of signal-to-noise ratio (SNR) on the measurements of kin using both diffusion methods, random noise was added to simulated signals according to the approach described previously [34]. A wide range of SNR from 20 to ∞, and 500 trials at each SNR level were simulated for each method.
Data analysis
All data analyses were performed using Matlab (Mathworks, Natick, MA, USA). For the CG experiments, the ADC of the slowly decaying component (i.e., DB) was determined by linear regression using the last 10 data points. Note that the logarithms of MR signals corresponding to those data points are linearly dependent on b when b values are very high, and hence the number of data points chosen in data analyses has minor influence on the results (see Figure 2A). The intracellular exchange lifetime τin and transcytolemmal water exchange rate constant kin were then derived from Eq. (4). For the FEXI experiments, a region of interest (ROI) was manually drawn on the central image, and ADC' values at each mixing time were calculated by fitting the ROI mean signals to exponential functions of the b value (S = S0 exp(−b·ADC')). The ADC' measured with an inactive filter (bf = 0 s/mm2) was regarded as that at infinite mixing time. AXR was determined by fitting ADC' as a function of tm according to Eq. (6). Typical SNR was over 1000 for the CG measurements and > 200 for the FEXI measurements.
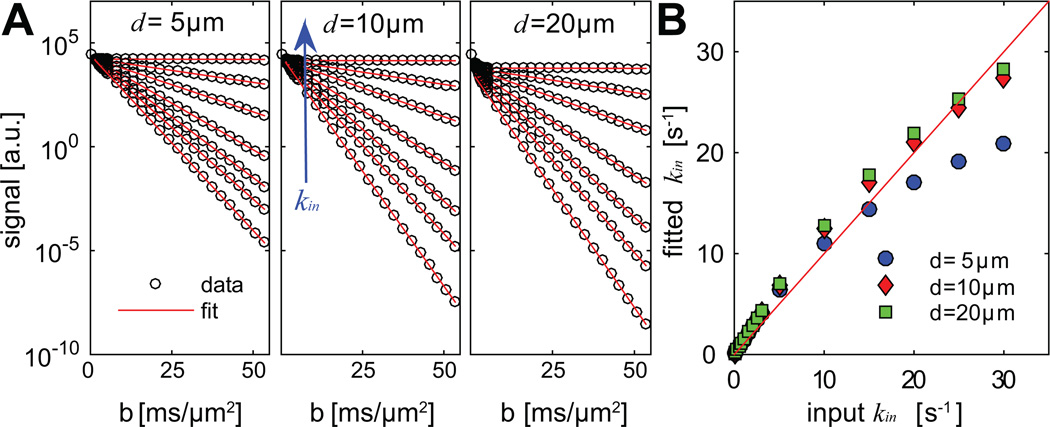
Figure 2.
Computer simulated non-normalized signals from CG-experiments arising from three different cell diameters (A) and a comparison of the corresponding fitted vs ground truth kin (B). Signals corresponding to seven kin values are shown in (A): 0.02, 2, 5, 10, 15, 20, and 30 s−1. Note that solid lines in (A) are used to better distinguish signals from different kin values.
RESULTS
Simulated dependency on kin and cell size
The simulated results using the CG method are shown in Figure 2. The logarithms of all diffusion-weighted signals show a linear dependence when b values are sufficiently high. The values of transcytolemmal water exchange rate constant kin were calculated based on DB and compared with the ground truth kin shown in Figure 2B. The influences of cell diameter are minor for d = 5, 10, and 20 µm when kin < 10 s−1. Meanwhile, the fitted kin values match well with the ground truth values in this kin range (r = 1 and p < 0.01 for all three d values provided by the Spearman correlation). Figure 2B shows a non-linearity between fitted and ground truth kin values. When kin > 15 s−1, there are large discrepancies between fitted and ground-truth kin, especially for the smallest d (5 µm). For example, the fitted kin is 30.6% lower when the ground-truth kin is 30 s−1, while the fitted kin is 8.9% and 5.9% lower for d = 10 and 20 µm. These underestimates occurred at higher kin values and may be due to the violation of the requirement of the CG method that the transcytolemmal water exchange should be slow enough [12]. Moreover, the CG method relies on the Gaussian phase approximation which may be invalid with high b values and small restriction sizes (e.g., cell size = 5 µm shown in Figure 2). Nevertheless, in the range of kin < 10 s−1, the CG method provides reasonable estimations of kin with minor influences from cell diameter. Note that kin < 10 s−1 is physiologically relevant in biological tissues i.e. intracellular water lifetime τin > 100 ms [32]. The non-linearity in Figure 2B suggests that the accuracy of the CG method in estimating kin is dependent on kin itself and cell size. It should be noted that, in the highly permeable regime kin > 15 s−1 such as in apoptotic regions, the biased estimation of kin limits the ability of the CG method to reliably characterize transcytolemmal water exchange.
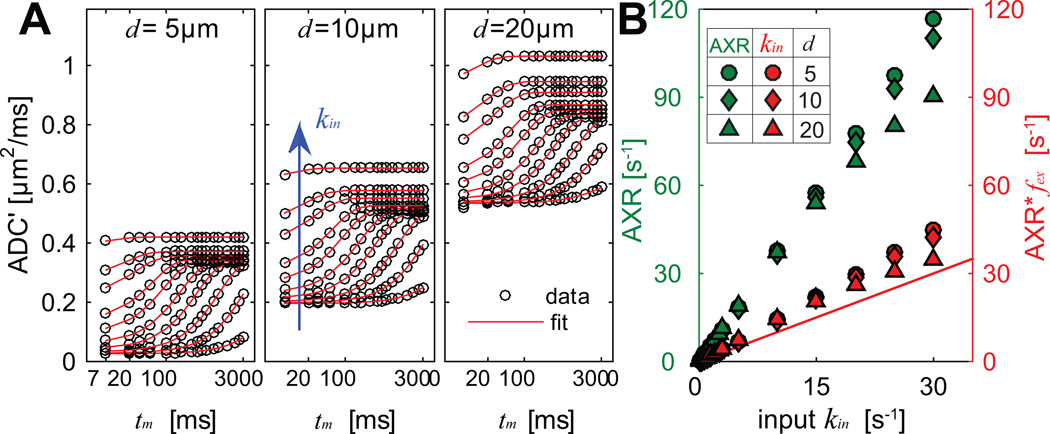
The simulated results using the FEXI method are shown in Figure 3. As expected, ADC' increases with tm, kin, and cell diameter d. All data are well described by Eq. (6). Comparisons of fitted AXR and kin = AXR × fex vs ground truth kin are provided in Figure 3B. The Spearman correlation provided r > 0.99 and p < 0.01 for any fitted AXR or kin with ground truth kin. The influences of cell sizes are minor when kin < 15 s−1, similar to the results using the CG method shown in Figure 2B. The discrepancies between fitted and true kin are significantly larger than those of the CG method: the fitted kin is 40% larger when true kin > 5 s−1, and 10% discrepancies occur only when true kin < 0.1 s−1 (τin > 10 sec, much longer than typical τin e.g. in normal brain [32]). This suggests the fitted kin is overestimated using the FEXI method even with the correction for the effects of fex. However, both AXR and fitted kin show approximately linear dependences on kin, indicating AXR still provides a promising means to assess kin non-invasively.
Figure 3.
Computer simulated ADC’ from FEXI experiments of three different cell diameters (A) and the comparison of AXR, fitted kin vs ground truth kin (B). ADC’ corresponding to ten kin values were shown in (A): 0.02, 0.1, 0.3, 0.75, 1.5, 3, 5, 10, 15, and 30 s−1. The x-axis is on logarithmic scale in (A), and solid lines in (A) are used to better distinguish signals from different kin values.
Simulated influence of SNR
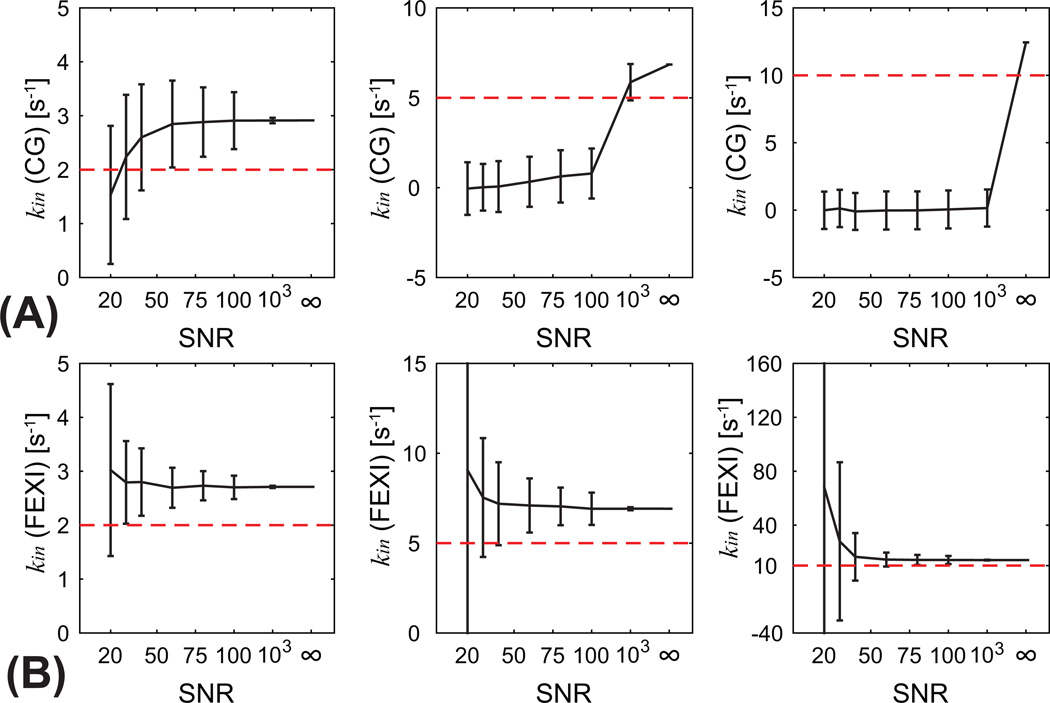
Figure 4 shows the simulated influence of SNR on the measurements of kin using CG and FEXI methods for a typical cancer cell size d = 10 µm and three ground truth kin values: 2, 5, and 10 s−1. Under such circumstances, both CG and FEXI methods overestimate kin even when SNR = ∞, although the CG method provide slightly more accurate result especially for ground truth kin = 5, or 10 s−1. For example, when ground truth kin = 5 s−1, the CG method estimates kin of 6.85 s−1, < 1% more accurate than kin of 6.91 s−1 estimated using the FEXI method. However, the noise influence on both methods is remarkably different. For ground truth kin = 5 or 10 s−1, the CG method estimated kin all close to zeros when SNR is smaller than 100. This is because diffusion-weighted signals are under noise floor with very high b values, so that the CG method fails to estimate kin. For example, Figure 4 shows normalized signals at high b values are very small, i.e., < 1e−4, so that even SNR = 100 is not sufficient to estimate kin reliably. Under such circumstances, the linear fitting is dominated by noise and even negative kin values might be obtained. However, reasonable kin values can be obtained from the fittings when ground truth kin = 2 s−1, although deviated by ~ 1 s−1. This is because when transcytolemmal water exchange rate constant is slow (e.g. τin = 500 ms), the signals were mainly dominated by intracellular restricted diffusion which decays much slower with respective to b values. By contrast, kin (= AXR*fex) obtained using the FEXI method can estimate kin reliably for all ground truth kin values when SNR is sufficiently high (e.g. > 75). This suggests that the FEXI method is more robust at low SNRs which makes it possible for practical imaging, while the CG method typically requires very high SNR which makes it only suitable for large volume-of-interest in a non-imaging manner.
Figure 4.
The simulated influence of SNR on CG (A) and FEXI (B) methods for cell size d = 10 µm and three ground truth kin values: 2 (left column), 5 (middle), and 10 (right) s−1. Red dashed lines present ground truth values.
MR measurements of kin
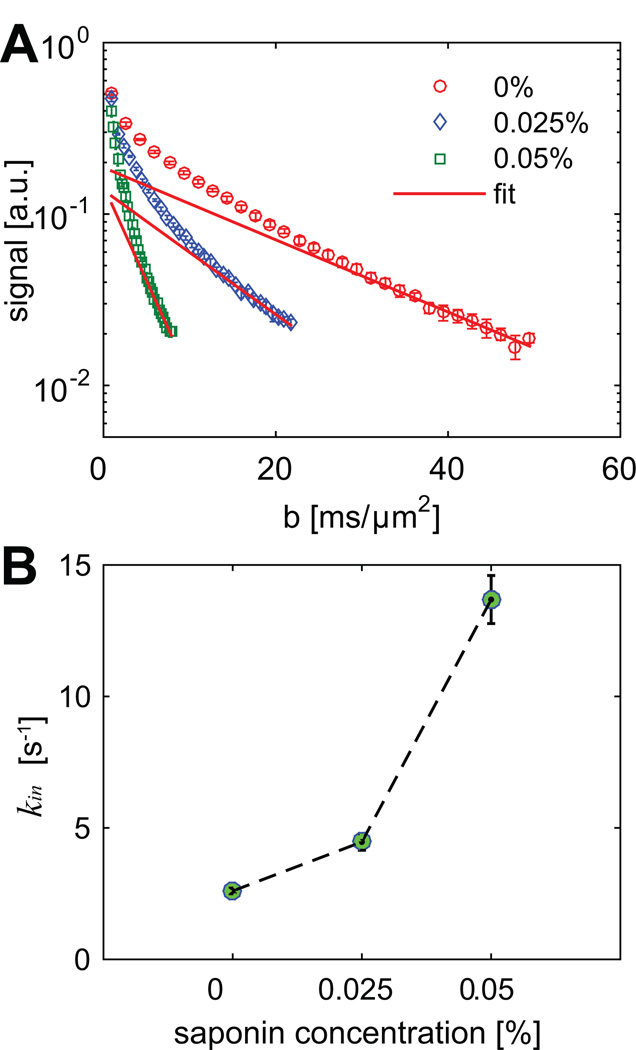
Figure 5 shows the measured signals and fitted kin obtained in the CG measurements with three different saponin concentrations: 0, 0.025, and 0.05%. Note that most error bars are smaller than symbol sizes in Figure 5. As shown in Figure 5B, measured kin increases significantly with higher concentration of saponin (r = 0.94 and p < 0.01). Recall that the ten signal data points with highest b values corresponding to each saponin concentration are used to fit kin, which increases with higher concentrations of saponin when cell membranes are more permeable. Note that SNR in the CG measurements are over several thousands, and hence the influence of noise is negligible so that relatively higher kin can be fitted, which is different from the results shown in Fig.4 with SNR < 100.
Figure 5.
The measured diffusion signals (A) and fitted kin values (B) from CG experiments vs three different saponin concentrations. The error bars in both sub-figures represent standard deviations across all cell samples.
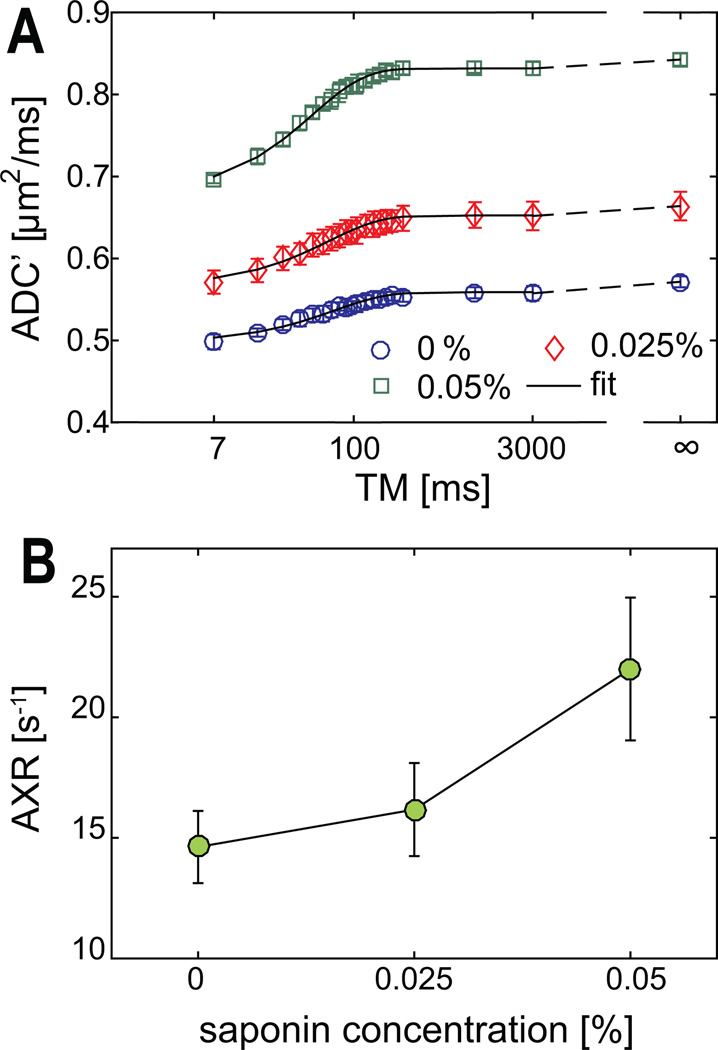
Figure 6 shows the measured ADC' and AXR values obtained from FEXI experiments of the same cell samples shown in Figure 5. All ADC' values are well described by the fits of Eq. (6). The fitted AXR’s increase significantly (r = 0.83 and p < 0.01) with higher concentrations of saponin as expected. Note that for the current experiments, only transmembrane permeability is expected to change with saponin concentration, indicating the cell membrane permeability changes have been detected by AXR. Note that the measured ADC at equilibrium (i.e., ADC' at infinite tm) increases with higher concentrations of saponin, presumably due to the increased cross-membrane permeability. This is consistent with our previous study that the variations of cross-membrane permeability alone can cause significant ADC changes [20].
Figure 6.
The measured ADC’ (A) and AXR values (B) from FEXI experiments vs three different saponin concentrations. The error bars in both sub-figures represent standard deviations across all cell samples. The x-axis is on logarithmic scale in A.
Experimental correlation between results from CG and FEXI methods
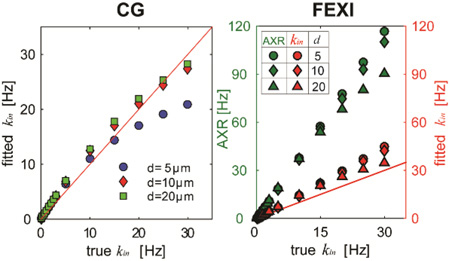
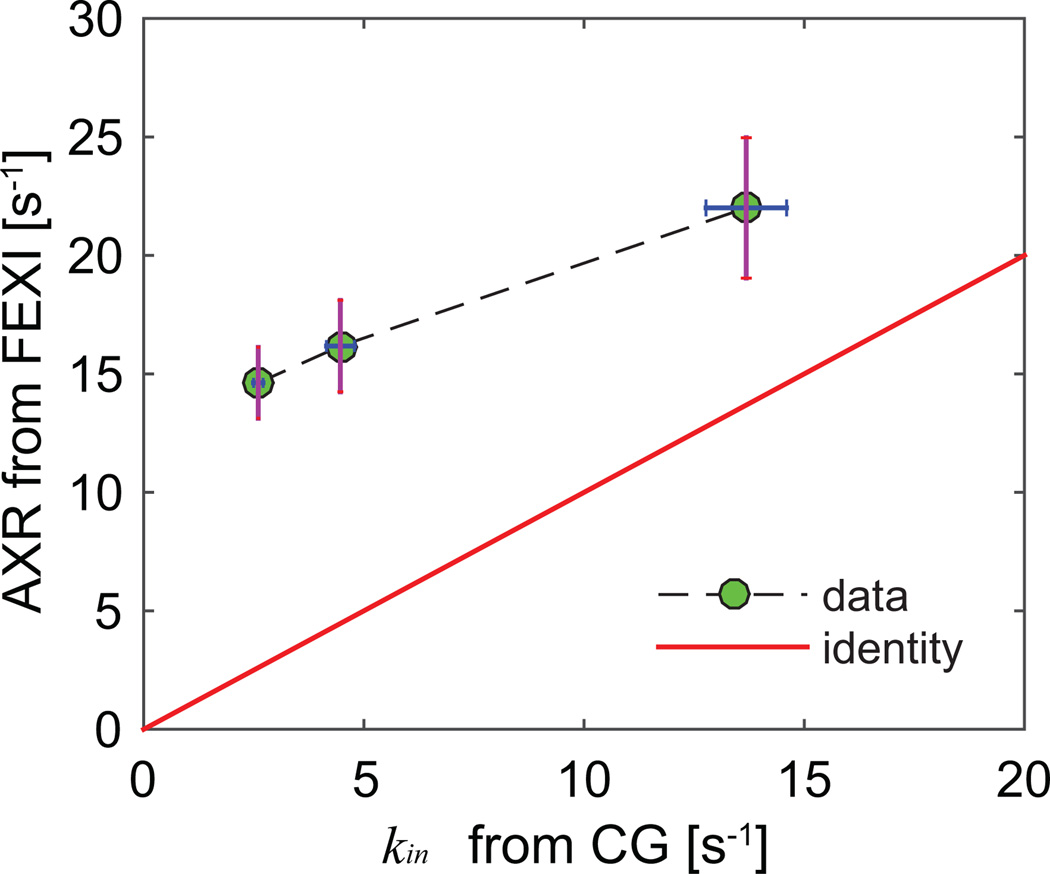
Figure 7 shows the correlation between AXR measured from the FEXI measurements vs kin from the CG experiments. AXR from the FEXI measurements are significantly higher than kin obtained using the CG methods due to the influence of extracellular water fraction fex. Compared with the simulated correlations shown in Figure 4, the discrepancies between AXR and kin from the CG method are much larger, presumably due to the larger fex values in vitro compared with those used in the simulations. Nevertheless, AXR and kin from the CG method are correlated in an approximately linear manner (r = 0.75 and p < 0.01), which implies the FEXI method may provide similar information as the CG method to probe the variations of transcytolemmal water exchange.
Figure 7.
The correlation between AXR measured from FEXI vs kin from CG experiments. Each point represents the mean value from all cell samples treated with the same saponin concentration, and the error bars represent corresponding standard deviations.
DISCUSSION
The transcytolemmal water exchange rate constant kin is not only an important indicator of the status of living cells, but also has a major influence on many MR measurements other than diffusion-weighted MR. For examples, it has been reported that kin needs to be incorporated in DCE-MRI models for more accurate estimations of parameters when kin is not fast enough [35]. Recent studies have also found relatively fast kin values are essential for the validity of widely-used two-pool models to describe quantitative magnetization transfer (qMT) [36] and chemical exchange saturation transfer (CEST) [37] measurements. Therefore, a good estimate of kin may not only provide a way to better characterize biological tissues, but also provides insights to better design experiments and data interpretation approaches for other MR methods.
Saponin used in our experiments has a concentration range of 71.4 to 223.2nM (based on the manufacturer provided data of 8–25% effective Sapogenin in their reagent), corresponding to 489 to 1527 saponin molecules per cell if they all interact with cell membranes. If saponin simply reaches equilibrium based on cell volume, there are 15 to 46 Saponin molecules per cell. This is certainly underestimated because there is a tendency for saponin to get into membranes due to the hydrophobicity nature. Hence, the actual number is estimated between 15 to 1527 saponin molecules per cell. By comparison, each mammalian cell has about 5×1010 lipids, and approximately a half is cholesterol. Therefore, the ratio of the number of saponin molecules to cell membrane cholesterol/lipid is extremely low. It is conceivable that, except increasing membrane permeability by removing a small number of cholesterols, the effect of saponin on other cell membrane structure is negligible at such a low concentration. This is consistent with previous studies that used detergents to change membrane permeability of fixed cells [26]. Note that Ref. [26] did not specifically measured cross-membrane water permeability, but found the enhanced permeability to large molecules such as oligonucleotides. Because water molecules are much smaller than oligonucleotides, we can reasonably infer that passive water permeability across fixed cell membrane increases after saponin treatment. This is consistent with our observation in this work.
The current work focuses on computer simulations and in vitro cell studies to generate varying transmembrane permeabilities that are within the physiologically relevant range. It should be noted that it is extremely challenging to fully mimic the cell environment in vivo. The fixation used in the current work removes vitality of cells which affects the membrane permeability significantly. However, an advantage of the current approach is that, with well-controlled conditions such as low concentrations of detergents, only membrane permeability is expected to be altered with minimal other side effects on fixed membrane structure. This is an advantage over the method of treating living cells using detergents, which may cause concurrent other effects that may confound the measurements and decrease the specificity to membrane permeability. Note that it may be more difficult for saponin to remove cholesterols on the surfaces of fixed cells because the membrane fluidity is expected to be greatly hindered after fixation. Nevertheless, our results suggest that the usage of saponin does increase the passive cross-membranes diffusion so that the overall averaged apparent transcytolemmal water exchange rate constant varies in a broad physiologically relevant range. Note that diffusion MR methods do not have the sensitivity to differentiate different types of water transport but characterize an overall averaged transcytolemmal water exchange rate constant. Therefore, even if our experimental settings cannot fully duplicate the in vivo environment, especially the inability to mimic active transport, our simplified tissue model system provides a testing framework to evaluate and compare both diffusion MR methods to characterize changes in membrane permeability.
The movement of molecules across a cell membrane can be either active or passive. The random diffusion is one main type of passive transport of water molecules, and there are other main types of water transport such as osmosis (caused by a cross-membrane osmotic gradient) and facilitated diffusion (transported by other molecules) [1]. It is important to emphasize that both diffusion MR methods used in the present work characterize an averaged cross-membrane permeability with an assumption of equilibrium water exchange. It is very challenging for the diffusion MRI methods to characterize the osmotic cross-membrane permeability with a dynamic net water transport. However, the dynamic net water transport may occur relatively slowly in some diseases. For example, it has been reported that it takes about 24 hours for extracellular space decreases from 20% to 4 – 10% after stroke [38, 39], much longer than typical scan time such as 45 mins for FEXI in vivo [4]. Under such circumstances, the apparent transcytolemmal water exchange rate constant is mainly dominated by diffusion or facilitated diffusion process under an assumption of close-to-equilibrium, which can be characterized using diffusion MR methods.
The CG (constant gradient) method has been developed for decades to measure an averaged transcytolemmal water exchange rate constant kin [11, 12, 14]. However, such a method has not been comprehensively validated for 3D structures. A previous in vitro study used the water channel blocker pCMBS to decrease cell membrane permeability Pm [14], but an increase of Pm is more physiologically relevant in various diseases in practice [3, 5, 6, 20]. In addition, the use of pCMBS on living cells may cause other concurrent microstructural and physiological variations. The present study used saponin on fixed cells in vitro to selectively increase Pm without causing other microstructural variations [20]. There was one previous computer simulation study of the CG method performed on 2D cylindrical tissues with τin from 231 to ∞ ms [12], without considering faster transcytolemmal water exchange (τin < 100 ms) which occurs in many diseases [3, 4]. A large range of τin from 33 ms to 50 sec was simulated in the current study to investigate the accuracy of the CG method to measure kin. The results suggest that the CG method provides a reasonable estimation of kin especially when kin < 10 s−1, corresponding to τin > 100 ms which is in the physiological range of normal tissues. For kin > 10 s−1, the kin obtained using the CG method still increases monotonically with ground truth kin, suggesting the CG method provides useful information on kin even with fast transcytolemmal water exchange which violates the requirements of the CG method. Because very high b values are necessary in the CG method which results in SNRs that are too low for high spatial resolution imaging, the CG method is preferred to detect kin of a large volume-of-interest spectroscopically when spatial mapping is not required.
Other than by heating [15] and detergent [17] treatment studies, the FEXI method has not been fully validated up to date. The dependence of AXR on both kin and fex makes it difficult to interpret FEXI data. The computer simulations used in the current study provide an opportunity to investigate the precise relation between true and fitted kin values using the FEXI method. Compared with the CG method, our simulations suggest that the FEXI method provides slightly less accurate results about transcytolemmal water exchange even after correction for the effects of fex at very high SNRs. Our simulations show that FEXI overestimates kin, which may be because the simulations and experiments violate some assumptions used in deriving Eq. (6) [4, 15, 17]. For example, the b = 1500 s/mm2 used in the diffusion detection block yields non-mono-exponential behavior for smaller cell sizes (5 µm), which violates the small b assumption to derive Eq. (6). Our simulations suggest this can lead to an overestimation kin. Moreover, the influences of non-uniform relaxation were not considered in the simulations, which may further increase the discrepancies between fitted and true kin. Despite these disadvantages, the FEXI method does not require extremely high SNR’s as the CG method. The fitted AXR and kin values obtained in computer simulations showed approximately linear dependence on ground truth kin (see Figure 3), indicating they still provide a reasonable means to measure kin. In the cell measurements in vitro with sufficiently high SNR’s, fitted AXR values correlated well with kin fitted using the CG method, suggesting they may provide similar information on variations of transcytolemmal water exchange rate constant. It should be pointed out that the increased cell membrane permeability Pm found in pathological tissues will cause larger errors in AXR, and the possibly variable fex will further complicate the interpretation of FEXI data in practice. As shown in Fig.2 and Fig.3, with higher kin values (i.e., fast exchange), the fitted kin deviates further from ground truth values in the simulations. This indicates the influences of the violation of slow exchange approximation in both methods. However, both methods provide reasonably well estimation of kin when kin < 10 s−1, which is typical for biological tissues. It should be prudent to interpret data from highly permeable tissues such as apoptotic regions, in which kin could be > 15 s−1 [3]. Nevertheless, the FEXI method provides a flexible and non-invasive imaging method capable of spatially mapping kin in humans [4, 16], which may have considerable clinical potential.
For the CG method, two conditions need to be satisfied [24], i.e. 1) a sufficiently long diffusion time is used, i.e. 4·tD·Din / d2 >>1, and 2) the transcytolemmal water exchange rate constant is slow, i.e. q̃2Dex >> kin. Note that very high b values are required in CG measurements with a fixed gradient strength (as well as the q value) so that the diffusion times are usually very long to meet the first condition. Meanwhile, the fixed gradient duration is much shorter than long diffusion times, and hence we do not expect the gradient duration to affect the CG measurements significantly. As shown in Fig.2, the CG method shows different accuracy with different kin values, i.e., more accurate with kin < 10 s−1 than those with kin > 15 s−1. This discrepancy is presumably due to how the slow exchange approximation is satisfied. Therefore, it is important to note that CG measurements may provide biased results when transcytolemmal water exchange rate constant is too large, such as in apoptotic regions.
For the FEXI method, the diffusion times used in either the filter or the detection PGSE blocks are chosen in such a way that significant transcytolemmal water exchange is expected to occur mainly during the mixing time and not much during each of the PGSE blocks. Ideally, the diffusion time tD should be much smaller than mixing time tm. Therefore, shorter diffusion times are preferred, which also limits the choices of gradient durations. Note that both diffusion times and gradient durations are fixed, and only mixing time tm is allowed to vary in FEXI measurements. Therefore, we do not expect slightly different diffusion times and gradient durations in practical ranges would significantly affect the FEXI results. It is of interest to investigate the exact influence of different choices of diffusion times and gradient durations on FEXI measurements, but is out of the main scope of current work.
It is desirable to measure intra- or extracellular water fraction fin or fex = 1 − fin and use this information to correct AXR values obtained in FEXI measurements for a better estimation of kin. DCE-MRI and diffusion-weighted MRI methods have been proposed to measure fin non-invasively. However, recent studies have found the accuracy of the measurements of fin are significantly affected by kin themselves. A recent study [40] reports that fin values obtained from either quantitative PGSE (pulsed gradient spin echo) [41] or OGSE (oscillating gradient spin echo) [42, 43] diffusion sequences are significantly underestimated due to transcytolemmal water exchange, while fitted cell size values are less influenced. Another study found out that “DCE-MRI is not a reliable biomarker of extracellular space for a range of physiological conditions” confirmed with histology analysis [44]. These findings demonstrate the difficulties of reliably estimating fin or fex in vivo, making it even more challenging to directly measure kin using the FEXI method.
Previous water exchange studies with red blood cells typically use Gd to “selectively” change extracellular relaxation properties [45]. The estimated kin has been reported as 52.4 s−1, much larger than the 2.6 s−1 of untreated K562 cells in the current study. This is anticipated because the mean K562 cell diameter is ~19 µm [20], much larger than typical red blood cell size of 6 – 8 µm. Note that the Gd-based method is also model based and has its own limitations. It has been reported that Gd-based contrast agents may introduce magnetic susceptibility gradients which cross compartmental boundaries and alter R2 measurements [32], which in turn effectively change relative water fractions. Even if R1 measurements have been widely used to estimate water exchange rate constant [32, 46], the contamination of MT on R1 measurements may significant alter the fitted water exchange rate constant, as shown by Prantner et al. [47]. Due to a lack of “gold standard” method to accurately quantify the transcytolemmal water exchange rate constant non-invasively, it is challenging to fully validate the accuracy of the relaxation- and diffusion-based methods to estimate kin.
The current study focuses on the evaluation and comparison of diffusion MR methods for measuring transcytolemmal water exchange rate constant kin using computer simulations and cultured cells in vitro with varying cell transmembrane permeabilties. Note that other biological tissues properties (such as relaxation and diffusion rates of intra- and extracellular space, cell shape, and diffusion anisotropy [19]) and acquisition strategies (such as choices of readout sequences) might influence the measurements of kin as well. The comprehensive investigation of all these influences on kin measurements is desirable in future studies.
CONCLUSIONS
Two diffusion-based methods, CG (constant gradient) and FEXI (filtered exchange imaging) methods, were comprehensively evaluated and compared using both computer simulations and well-controlled cultured cells with varying cell transmembrane permeabilties. The results suggest that, with sufficient SNR’s, the CG method provides reasonably accurate estimations of transcytolemmal water exchange rate constant kin when it is smaller than 10 s−1, which is in the physiological range of many biological tissues. However, the CG method requires high SNR which makes it applicable only with large volumes-of-interest. Although the FEXI method constantly overestimates kin even with corrections for the effects of extracellular water fraction, it provides reliable estimates with practical SNR’s and more importantly, results from the FEXI method agrees well with those from the CG method with high SNR’s experimentally. Considering the feasibility of spatially mapping kin in humans in vivo, the FEXI method is of clinical potential as shown recently although its absolute accuracy is compromised.
Highlights.
Both CG or FEXI methods are sensitive to transcytolemmal water exchange rate constant kin
The CG method provide accurate estimation of kin with high SNR and kin < 10 s−1
The FEXI method overestimates kin but agrees with the CG method experimentally
The FEXI method might have greater clinical potential compared with the CG method
Acknowledgments
This work was funded by NIH grants K25CA168936; R01CA109106; R01CA173593.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Amiry-Moghaddam M, Ottersen OP. The molecular basis of water transport in the brain. Nat Rev Neurosci. 2003;4:991–1001. doi: 10.1038/nrn1252. [DOI] [PubMed] [Google Scholar]
- 2.Day RE, Kitchen P, Owen DS, Bland C, Marshall L, Conner AC, Bill RM, Conner MT. Human aquaporins: regulators of transcellular water flow. Biochim Biophys Acta. 2014;1840:1492–1506. doi: 10.1016/j.bbagen.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 3.Bailey C, Moosvi F, Stanisz GJ. Mapping water exchange rates in rat tumor xenografts using the late-stage uptake following bolus injections of contrast agent. Magn Reson Med. 2014;71:1874–1887. doi: 10.1002/mrm.24847. [DOI] [PubMed] [Google Scholar]
- 4.Nilsson M, Latt J, van Westen D, Brockstedt S, Lasic S, Stahlberg F, Topgaard D. Noninvasive mapping of water diffusional exchange in the human brain using filter-exchange imaging. Magn Reson Med. 2013;69:1573–1581. doi: 10.1002/mrm.24395. [DOI] [PubMed] [Google Scholar]
- 5.Volles MJ, Lansbury PT. Vesicle permeabilization by protofibrillar alpha-synuclein is sensitive to Parkinson's disease-linked mutations and occurs by a pore-like mechanism. Biochemistry. 2002;41:4595–4602. doi: 10.1021/bi0121353. [DOI] [PubMed] [Google Scholar]
- 6.Moftakhar P, Lynch MD, Pomakian JL, Vinters HV. Aquaporin expression in the brains of patients with or without cerebral amyloid angiopathy. Journal of neuropathology and experimental neurology. 2010;69:1201–1209. doi: 10.1097/NEN.0b013e3181fd252c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farinas J, Kneen M, Moore M, Verkman AS. Plasma membrane water permeability of cultured cells and epithelia measured by light microscopy with spatial filtering. J Gen Physiol. 1997;110:283–296. doi: 10.1085/jgp.110.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perazella MA. Current status of gadolinium toxicity in patients with kidney disease. Clin J Am Soc Nephrol. 2009;4:461–469. doi: 10.2215/CJN.06011108. [DOI] [PubMed] [Google Scholar]
- 9.Karger J, Pfeifer H, Heink W. Principles and applications of self-diffusion measurements by nuclear magnetic resonance. In: Waugh JS, editor. Advances in Magnetic Resonance. 1250 Sixth Avenue San Diego, California 92101: Academic Press, Inc; 1988. pp. 1–89. [Google Scholar]
- 10.Price WS, Barzykin AV, Hayamizu K, Tachiya M. A model for diffusive transport through a spherical interface probed by pulsed-field gradient NMR. Biophysical Journal. 1998;74:2259–2271. doi: 10.1016/S0006-3495(98)77935-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfeuffer J, Flogel U, Dreher W, Leibfritz D. Restricted diffusion and exchange of intracellular water: theoretical modelling and diffusion time dependence of 1H NMR measurements on perfused glial cells. NMR Biomed. 1998;11:19–31. doi: 10.1002/(sici)1099-1492(199802)11:1<19::aid-nbm499>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 12.Meier C, Dreher W, Leibfritz D. Diffusion in compartmental systems. I. A comparison of an analytical model with simulations. Magn Reson Med. 2003;50:500–509. doi: 10.1002/mrm.10557. [DOI] [PubMed] [Google Scholar]
- 13.Meier C, Dreher W, Leibfritz D. Diffusion in compartmental systems. II. Diffusion-weighted measurements of rat brain tissue in vivo and postmortem at very large b-values. Magn Reson Med. 2003;50:510–514. doi: 10.1002/mrm.10558. [DOI] [PubMed] [Google Scholar]
- 14.Pfeuffer J, Flogel U, Leibfritz D. Monitoring of cell volume and water exchange time in perfused cells by diffusion-weighted 1H NMR spectroscopy. NMR Biomed. 1998;11:11–18. doi: 10.1002/(sici)1099-1492(199802)11:1<11::aid-nbm498>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 15.Aslund I, Nowacka A, Nilsson M, Topgaard D. Filter-exchange PGSE NMR determination of cell membrane permeability. J Magn Reson. 2009;200:291–295. doi: 10.1016/j.jmr.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Lasic S, Oredsson S, Partridge SC, Saal LH, Topgaard D, Nilsson M, Bryskhe K. Apparent exchange rate for breast cancer characterization. NMR Biomed. 2016;29:631–639. doi: 10.1002/nbm.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lasic S, Nilsson M, Latt J, Stahlberg F, Topgaard D. Apparent exchange rate mapping with diffusion MRI. Magn Reson Med. 2011;66:356–365. doi: 10.1002/mrm.22782. [DOI] [PubMed] [Google Scholar]
- 18.Lampinen B, Szczepankiewicz F, van Westen D, Englund E, Latt CSPJ, Stahlberg F, Nilsson M. Optimal experimental design for filter exchange imaging: Apparent exchange rate measurements in the healthy brain and in intracranial tumors. Magn Reson Med. 2016 doi: 10.1002/mrm.26195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonderby CK, Lundell HM, Sogaard LV, Dyrby TB. Apparent exchange rate imaging in anisotropic systems. Magn Reson Med. 2014;72:756–762. doi: 10.1002/mrm.24957. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Jiang X, Xie J, McIntyre JO, Gore JC, Xu J. Time-Dependent Influence of Cell Membrane Permeability on MR Diffusion Measurements. Magn Reson Med. 2016;75:1927–1934. doi: 10.1002/mrm.25724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ash R, Barrer RM, Craven RJB. Sorption kinetics and time-lag theory. Part 1. Constant diffusion coefficient. Journal of the Chemical Society, Faraday Transactions II. 1978;74:40. [Google Scholar]
- 22.Jespersen SN, Pedersen M, Stødkilde-Jørgensen H. The influence of a cellular size distribution on NMR diffusion measurements. European Biophysics Journal. 2005;34:890–898. doi: 10.1007/s00249-005-0459-y. [DOI] [PubMed] [Google Scholar]
- 23.Sehy JV, Banks AA, Ackerman JJ, Neil JJ. Importance of intracellular water apparent diffusion to the measurement of membrane permeability. Biophys J. 2002;83:2856–2863. doi: 10.1016/S0006-3495(02)75294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fieremans E, Novikov DS, Jensen JH, Helpern JA. Monte Carlo study of a two-compartment exchange model of diffusion. NMR in Biomedicine. 2010;23:711–724. doi: 10.1002/nbm.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seeman P, Cheng D, Iles GH. Structure of membrane holes in osmotic and saponin hemolysis. J Cell Biol. 1973;56:519–527. doi: 10.1083/jcb.56.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amidzadeh Z, Behbahani AB, Erfani N, Sharifzadeh S, Ranjbaran R, Moezi L, Aboualizadeh F, Okhovat MA, Alavi P, Azarpira N. Assessment of different permeabilization methods of minimizing damage to the adherent cells for detection of intracellular RNA by flow cytometry. Avicenna J Med Biotechnol. 2014;6:38–46. [PMC free article] [PubMed] [Google Scholar]
- 27.Xu J, Does MD, Gore JC. Numerical study of water diffusion in biological tissues using an improved finite difference method. Phys Med Biol. 2007;52:N111–N126. doi: 10.1088/0031-9155/52/7/N01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang SN, Chin CL, Wehrli FW, Hackney DB. An image-based finite difference model for simulating restricted diffusion. Magn Reson Med. 2003;50:373–382. doi: 10.1002/mrm.10536. [DOI] [PubMed] [Google Scholar]
- 29.Xu J, Does MD, Gore JC. Sensitivity of MR diffusion measurements to variations in intracellular structure: effects of nuclear size. Magn Reson Med. 2009;61:828–833. doi: 10.1002/mrm.21793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holz M, Heil SR, Sacco A. Temperature-dependent self-diffusion coefficients of water and six selected molecular liquids for calibration in accurate 1H NMR PFG measurements. Phys Chem Chem Phys. 2000;2:4740–4742. [Google Scholar]
- 31.Ackerman JJH, Neil JJ. The use of MR-detectable reporter molecules and ions to evaluate diffusion in normal and ischemic brain. NMR in biomedicine. 2010;23:725–733. doi: 10.1002/nbm.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quirk JD, Bretthorst GL, Duong TQ, Snyder AZ, Springer CS, Jr, Ackerman JJ, Neil JJ. Equilibrium water exchange between the intra- and extracellular spaces of mammalian brain. Magn Reson Med. 2003;50:493. doi: 10.1002/mrm.10565. [DOI] [PubMed] [Google Scholar]
- 33.Novikov DS, Jensen JH, Helpern JA, Fieremans E. Revealing mesoscopic structural universality with diffusion. Proc Natl Acad Sci U S A. 2014;111:5088–5093. doi: 10.1073/pnas.1316944111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- 35.Yankeelov TE, Luci JJ, DeBusk LM, Lin PC, Gore JC. Incorporating the effects of transcytolemmal water exchange in a reference region model for DCE-MRI analysis: theory, simulations, and experimental results. Magn Reson Med. 2008;59:326–335. doi: 10.1002/mrm.21449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li K, Li H, Zhang XY, Stokes AM, Jiang X, Kang H, Quarles CC, Zu Z, Gochberg DF, Gore JC, Xu J. Influence of water compartmentation and heterogeneous relaxation on quantitative magnetization transfer imaging in rodent brain tumors. Magn Reson Med. 2016;76:635–644. doi: 10.1002/mrm.25893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H, Li K, Zhang XY, Jiang X, Zu Z, Zaiss M, Gochberg DF, Gore JC, Xu J. R1 correction in amide proton transfer imaging: indication of the influence of transcytolemmal water exchange on CEST measurements. NMR Biomed. 2015;28:1655–1662. doi: 10.1002/nbm.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia JH, Yoshida Y, Chen H, Li Y, Zhang ZG, Lian J, Chen S, Chopp M. Progression from ischemic injury to infarct following middle cerebral artery occlusion in the rat. The American Journal of Pathology. 1993;142:623–635. [PMC free article] [PubMed] [Google Scholar]
- 39.Liang D, Bhatta S, Gerzanich V, Simard JM. Cytotoxic edema: mechanisms of pathological cell swelling. Neurosurgical focus. 2007;22:E2-2. doi: 10.3171/foc.2007.22.5.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li H, Jiang X, Xie J, Gore JC, Xu J. Impact of transcytolemmal water exchange on estimates of tissue microstructural properties derived from diffusion MRI. Magn Reson Med. 2016 doi: 10.1002/mrm.26309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panagiotaki E, Walker-Samuel S, Siow B, Johnson SP, Rajkumar V, Pedley RB, Lythgoe MF, Alexander DC. Noninvasive quantification of solid tumor microstructure using VERDICT MRI. Cancer Res. 2014;74:1902–1912. doi: 10.1158/0008-5472.CAN-13-2511. [DOI] [PubMed] [Google Scholar]
- 42.Jiang X, Li H, Xie J, Zhao P, Gore JC, Xu J. Quantification of cell size using temporal diffusion spectroscopy. Magn Reson Med. 2016;75:1076–1085. doi: 10.1002/mrm.25684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu J, Li H, Harkins KD, Jiang X, Xie J, Kang H, Does MD, Gore JC. Mapping mean axon diameter and axonal volume fraction by MRI using temporal diffusion spectroscopy. Neuroimage. 2014;103:10–19. doi: 10.1016/j.neuroimage.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barnes SL, Sorace AG, Loveless ME, Whisenant JG, Yankeelov TE. Correlation of tumor characteristics derived from DCE-MRI and DW-MRI with histology in murine models of breast cancer. NMR Biomed. 2015;28:1345–1356. doi: 10.1002/nbm.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gianolio E, Ferrauto G, Di Gregorio E, Aime S. Re-evaluation of the water exchange lifetime value across red blood cell membrane. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2016;1858:627–631. doi: 10.1016/j.bbamem.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 46.Prantner AM, Bretthorst GL, Neil JJ, Garbow JR, Ackerman JJ. Magnetization transfer induced biexponential longitudinal relaxation. Magn Reson Med. 2008;60:555–563. doi: 10.1002/mrm.21671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prantner AM. Re-evaluation of Transmembrane Water Exchange in the Rat Brain. ProQuest. 2008 [Google Scholar]