Table 1.
Asymmetric Cyclopropanation of Styrene with Tosylhydrazones by Metalloradical Catalysts [Co(D2-Por*)]a
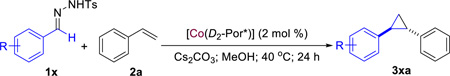 | ||||||
|---|---|---|---|---|---|---|
| entry | R | catalyst | product | yield (%)b | dec | ee (%)d |
| 1 | H (1a) | 3aa | 46 | 88:12 | - | |
| 2 | H (1a) | 3aa | 64 | 89:11 | −42 | |
| 3 | H (1a) | 3aa | 65 | 78:22 | <5 | |
| 4 | H (1a) | 3aa | 67 | 95:5 | 26 | |
| 5 | 2-MeO (1b) | 3ba | 78 | 95:5 | 99 | |
| 6 | 2-Et (1c) | 3ca | 87 | 85:15 | 33 | |
| 7 | 4-MeO (1d) | 3da | 23 | 93:7 | 23 | |
| 8 | 3-MeO (1e) | 3ea | 69 | 95:5 | 23 | |
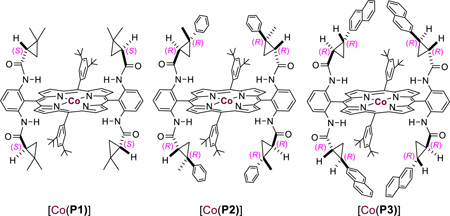 | ||||||
Carried out with 1x (0.1 mmol) and 2a (1.5 equiv.) in the presence of Cs2CO3 (2 equiv.) in methanol (0.6 mL).
Isolated yields.
Determined by 1H NMR.
Determined by chiral HPLC for the major trans diastereomer.
