Table 3.
Asymmetric Cyclopropanation of Styrene with Various Sulfonyl Hydrazones Catalyzed by [Co(P3)]a
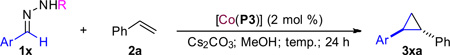 | |||||||
|---|---|---|---|---|---|---|---|
| entry |
|
product | temp. (°C) |
yield (%)c |
drd | ee (%)e |
|
| 1 | Ts(1b) | 3ba | 40 | 78 | 95:5 | 99 | |
| 2 | Ts(1f) | 3fa | 40 | 91 | 95:5 | 94 | |
| 3 | 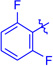 |
Ts(1g) | 3ga | RT | 90 | 94:6 | 86 |
| 4 | Ts (1g) | 3ga | 0 | <10 | - | - | |
| 5 | TPS (1g') | 3g'a | 0 | 83 | >99:1 | 93 | |
| 6 | Ts (1h) | 3ha | RT | 58 | 96:4 | 71 | |
| 7 | TPS (1h') | 3h'a | 0 | 75 | >99:1 | 76 | |
| 8 | 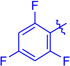 |
Ts (1i) | 3ia | RT | 85 | 96:4 | 88 |
| 9 | TPS (1i') | 3i'a | 0 | 85 | >99:1 | 93 | |
| 10 | 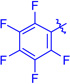 |
Ts (1j) | 3ja | RT | 82 | 95:5 | 68 |
| 11 | TPS (1j') | 3j'a | 0 | 81 | >99:1 | 89 | |
Carried out with 1x (0.1 mmol) and 2a (1.5 equiv.) in the presence of Cs2C O3 (2 equiv.) in methanol (0.6 mL).
Ts = 4-toluenesulfonyl; TPS = (2,4,6-triisopropyl) phenyl sulfonyl.
Isolated yields.
Determined by 1H NMR.
Determined by chiral HPLC for the major trans diastereomer.
