Abstract
The EuII/III redox couple offers metal-based oxidation-sensing with magnetic resonance imaging making the study of EuII oxidation chemistry important in the design of new probes. Accordingly, we explored oxidation reactions with a set of EuII-containing complexes. We report the observation of superoxide formation from the reaction between EuII and dioxygen using electron paramagnetic resonance spectroscopy. Additionally, we report oxidation kinetics of three EuII-containing complexes with bromate and glutathione disulfide at pH values including 5 and 7. In the reaction with bromate, the oxidation rate of two of the complexes increased by 7.3 and 6.7× upon decreasing pH from 7 to 5, but the rate increased by 17× for a complex containing amide functional groups over the same pH range. The oxidation rate of a fluorobenzo-functionalized cryptate was relatively slow, indicating that the ligand used to impart thermodynamic oxidative stability might also be useful for controlling oxidation kinetics.
Keywords: chelates, imaging agents, kinetics, lanthanides, redox chemistry
Graphical abstract
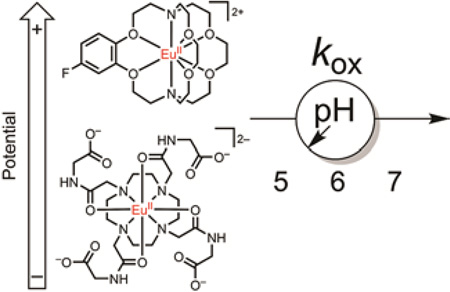
Release the electron: The oxidation chemistry of EuII-containing cyclen-, aqua-, and cryptand-based complexes was explored. The oxidation of EuII by BrO3− was faster at pH 5 than pH 7 for all complexes, but the oxidation rate of the cyclen-based tetraglycinate complex was relatively sensitive to changes in pH. A general lack of reactivity between EuII and glutathione disulfide was suggestive of activation barriers to the reaction over a 4 h period.
Introduction
The redox chemistry of lanthanides has received increasing attention over recent years because their +2, +3, and +4 oxidation states show promise for applications such as single-molecule and single-ion magnetism,[1] luminescence,[2] stoichiometric and catalytic reductions,[3] and redox-responsive magnetic resonance imaging (MRI).[4–7] Out of the lanthanide ions, EuII is uniquely poised for oxidation-responsive MRI because (1) some EuII-containing complexes are stable in aqueous media, (2) EuII provides positive (bright or T1-shortening) contrast enhancement in MRI, (3) the E1/2 of EuII/III can be tuned over a physiologically relevant range, and (4) the +2 and +3 oxidation states of Eu exhibit different magnetic and spectroscopic characteristics making responsive imaging feasible.[7,8] The oxidation-responsive behavior of EuII-containing complexes has been documented in vivo,[5,6] making the kinetics of oxidation under physiological conditions important to understand for the rational design of new complexes. Accordingly, there is a need for kinetic and thermodynamic analyses to enable a detailed understanding of EuII-containing complexes for responsive MRI. Here, we report that superoxide (O2−) is a product of the reaction between O2 and EuCl2 using a radical trap and electron paramagnetic resonance (EPR) spectroscopy. We also report the rates of oxidation of three EuII-containing complexes in the presence of bromate (BrO3−) and glutathione disulfide (GSSG) in aqueous media at pH 5 and 7.
Aqueous EuII oxidation chemistry for electron-transfer reactions has been explored within the context of Marcus theory for self-exchange redox reactions,[9–11] where the europium self-exchange (EuIIa + EuIIIb → EuIIIa + EuIIb) reaction rate (k ≤ 3 × 10−5 M−1 s−1) is slower than the corresponding FeII/III self-exchange reaction rate (k = 4 M−1 s−1).[10] One explanation for the discrepancy in self-exchange reaction rates involves the radial constriction of 4f orbitals relative to 3d orbitals.[10] A separate explanation invokes different energetic barriers for Eu and Fe self-exchange reactions imposed by solvent reorganizational energy.[10] Regardless of the differences between EuII/III and FeII/III self-exchange chemistry, the non-self-exchange oxidation of EuII is an important reaction to study because it can be used to help understand the behavior of EuII under physiological conditions. Non-self-exchange EuII electron-transfer has been explored using the EuII aqua ion in strongly acidic media.[12] Also in acidic media, the rate of oxidation of the EuII aqua ion by hydrogen peroxide was slowed by chelation of EuII with cyclic crown ethers and cryptands.[13] Studying the oxidation chemistry of chelated EuII under conditions representative of physiological conditions would be more relevant to redox-responsive MRI. We sought to build upon the observations in acidic solution by exploring the oxidation of EuII-containing complexes in buffered aqueous media. Before performing kinetic measurements, we studied a fundamental reaction between EuCl2 and O2 to determine if an oxygen-containing product could be identified. Given the in vivo correlation between tissue pO2 and the persistence of EuII-based positive contrast enhancement,[5,6] these studies are important for rationalizing EuII oxidation-responses.
Results and Discussion
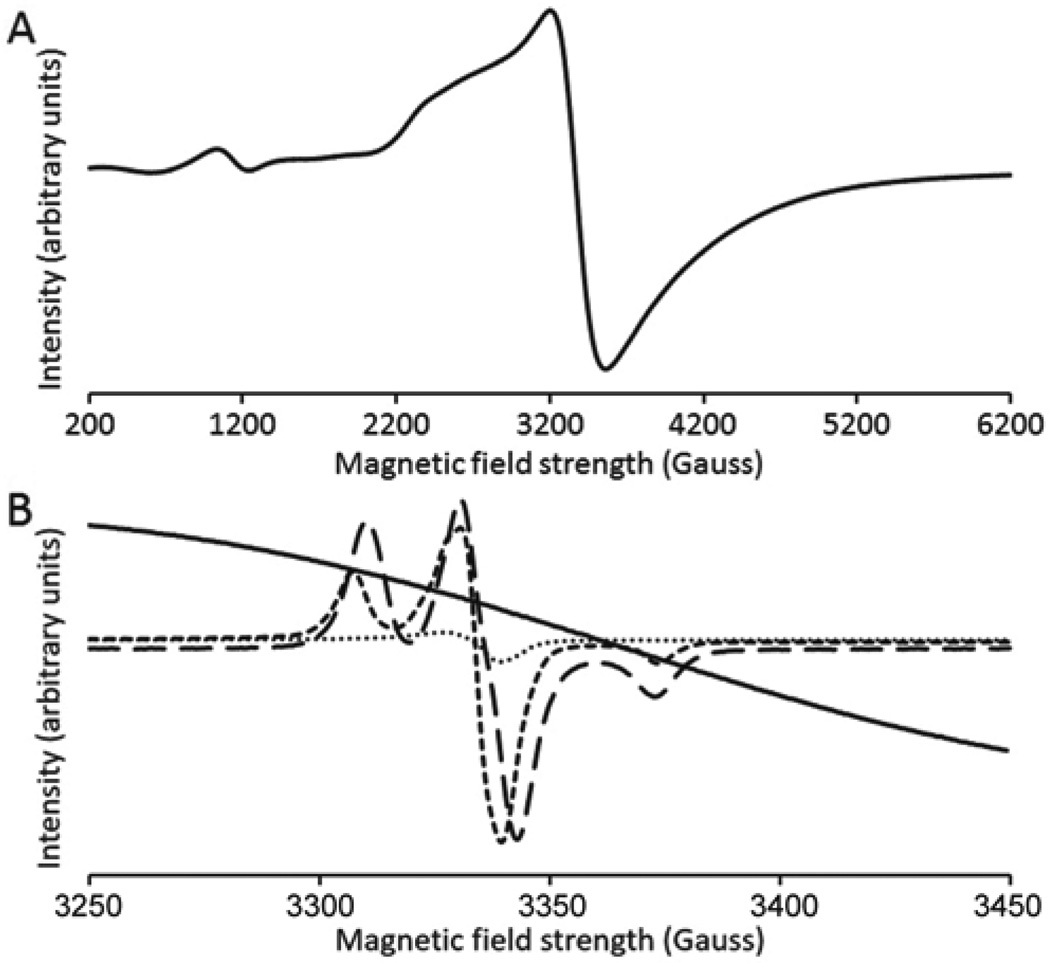
EuII is a one-electron reductant, and a one-electron reduction of O2 produces O2−. To gain insight into the oxidation of EuII by O2, we used the EPR spin trap 5-tert-butoxycarbonyl 5-methyl-1-pyrroline N-oxide (BMPO). BMPO is capable of reacting with O2− to form a relatively stable radical adduct that is observable with EPR spectroscopy.[14] To better our chances of observing a radical product, we used methanol as the solvent because O2− is more stable in non-aqueous than aqueous media.[15] Because we did not measure reaction kinetics, the use of physiological conditions was not necessary to identify a reaction product from EuCl2 exposed to O2. A direct reaction between EuCl2 and BMPO was not observed, but a radical signal was observed upon bubbling O2 into a solution containing EuCl2 and BMPO (Figure 1). When KO2 was added as a source of O2− to BMPO, an EPR profile nearly identical to the product of EuCl2 reacting with O2 was observed (Figure 1). However, the EPR spectrum of KO2 in the absence of BMPO did not produce the same EPR signal. The relatively small line shape differences between the EPR spectra of O2 reacting with EuCl2 in the presence of BMPO and KO2 in the presence of BMPO (Figure 1B) can be explained by the presence of EuIII ions in the former sample. EuIII has a diamagnetic ground state (7F0) and thermally accessible excited states, such as 7F1, that have nonzero effective magnetic moments. Population of the 7F1 excited state has been observed at 8 K,[16] and it is therefore reasonable that a paramagnetic excited state was populated to some extent at 110 K. Additionally, it could be envisioned that the hydroperoxyl–BMPO radical adduct coordinated to EuIII, and this interaction caused a relatively small perturbation in the chemical environment of the unpaired electron of the hydroperoxyl–BMPO radical adduct. When KO2 was mixed with BMPO in the presence of EuCl3 as a direct source of EuIII, a similar line shape difference was observed as from the reaction between EuCl2, O2, and BMPO (Figure S1). This experiment indicates that the line shape differences are due to the presence of EuIII. Regardless of the small differences between the spectra in Figure 1, these data suggest that O2− is a product of the reaction between O2 and EuCl2. After demonstrating the formation of O2−, we turned our attention to aqueous oxidation reactions for kinetic and thermodynamic analyses.
Figure 1.
EPR spectra of (A) EuCl2 + BMPO (wide view) and (B) EuCl2 + BMPO (—), EuCl2 + O2 + BMPO (— —), KO2 + BMPO (- -), and KO2 (· ·) in methanol at 110 K under Ar.
We measured the kinetics of oxidation of EuII-containing complexes 1-EuII, EuCl2(aq), and 2-EuII (Figure 2) because EuCl2(aq) is an aqueous analogue of EuCl2 used in our EPR studies, and 1-EuII and 2-EuII have been used for oxidation-responsive imaging.[5,6] Additionally, 1-EuII, EuCl2(aq), and 2-EuII encompass a relatively wide range in reduction potentials (Table 1) with 1-EuII being 374 mV more negative than EuCl2(aq)[7] and 2-EuII being 547 mV more positive than EuCl2(aq).[17] Although we have observed rapid oxidation of EuII by gaseous diatomic molecules, such as O2 and NO, we elected to study reaction kinetics with non-gaseous oxidants for the ease of quantifying and handling in an inert atmosphere. Because of our in vivo observations between pO2 and EuII oxidation and the EPR data with O2 presented in this work, we chose the anion BrO3− as a substitute for O2 for kinetic measurements. While structurally different, the relatively positive reduction potentials of BrO3− and O2 (1.06 and 0.815 mV vs normal hydrogen electrode,[18–20] respectively, at pH 7) indicate that BrO3− should, from a thermodynamic standpoint, spontaneously oxidize 1-EuII, EuCl2(aq), and 2-EuII. It should be noted that similarly positive reduction potentials do not equate to similar oxidation rates. GSSG was chosen as an oxidant because it is a major component of the cellular redox buffer along with reduced glutathione.[20] GSSG was expected to react spontaneously with 1-EuII and EuCl2(aq) but not react with 2-EuII based on Gibbs free energies calculated using electrochemical data (Table 1). We expected that a wide range in EuII reductant strength and ligand structure combined with relatively strong-to-intermediate oxidants would yield fundamental information regarding aqueous oxidation chemistry of EuII-containing complexes via comparison of reaction rates in aqueous media.
Figure 2.
Macrocyclic tetraglycinate complex 1-EuII; the EuII aqua ion, EuCl2(aq); and the 4-fluorobenzo-functionalized complex 2-EuII. Inner-sphere water and counterions have been omitted for clarity. Reported E1/2 values are versus normal hydrogen electrode and are below 1-EuII,[7], EuCl2(aq),[17] and 2-EuII.[17]
Table 1.
Estimated Gibbs free energies (kJ) for redox reactions involving 1-EuII, EuCl2(aq), and 2-EuII with BrO3− or GSSG at pH 5, 6, and 7.
| Reaction | BrO3−[d] (pH 5) |
BrO3−[d] (pH 6) |
BrO3−[d] (pH 7) |
GSSG[e] (pH 5) |
GSSG[e] (pH 7) |
|---|---|---|---|---|---|
| 1-EuII[a] | −1078[f] | −1043[f] | −1008 | −122[f] | −99 |
| EuCl2(aq)[b] | −861[f] | −826[f] | −792 | −50[f] | −27 |
| 2-EuII[c] | −545[f] | −510[f] | −475 | 56[f] | 79 |
1-EuIII + e− ⇌ 1-EuII (E = −0.682 V);[7]
EuIII + e− ⇌ EuII (E = −0.308 V);[17]
2-EuIII + e− ⇌ 2-EuII (E = 0.239 V);[17]
BrO3− + 6e− + 6H+ ⇌ Br− + 3H2O (EpH5 = 1.18 V, EpH6 = 1.12 V, EpH7 = 1.06 V);[17]
GSSG + 2e− + 2H+ ⇌ 2GSH (EpH5 = −0.051 V, EpH7 = −0.169 V);[20]
Calculated assuming a 59.1 mV change in reported potential per unit decrease in pH;[19] Estimated Gibbs free energies calculated using ΔG = −nFEcell where n is the number of electrons, F = 96,458 C mol−1, and Ecell = Ered + Eox. Reduction potentials are listed vs normal hydrogen electrode.
To measure the kinetics of oxidation of 1-EuII, EuCl2(aq), and 2-EuII by BrO3− and GSSG, loss of EuII was monitored using normalized UV–visible absorbance as a function of time (Figure 3). Experiments were conducted at pH 5, 6, and 7 because these values encompass a physiologically relevant range of pH values.[21] A reaction time of 240 min was chosen because it is longer than the clearance half-life (18 min) of small lanthanide-containing complexes from rats,[22] and therefore, represents a relevant period of time to observe oxidation events. Absorbance was measured at the maximum value for each complex: 353 nm for 1-EuII and 320 nm for EuCl2(aq) and 2-EuII (see Figure S2 for representative spectra). The absorption of UV–visible light at wavelengths longer than 300 nm was not observed for the EuIII-containing reaction products, indicating that decreases in absorbance are the result of oxidation of EuII. Oxidation rate (kox) data (Table 2) revealed the acceleration of EuII oxidation upon lowering the pH from 7 to 5 for every reaction with BrO3−, but oxidation was not observed for any EuII-containing complex at either pH with GSSG. Reactions with GSSG at pH 6 were not performed because no reactivity was observed at pH 5 or 7. There were increases of 1.4 and 2.3× in kox from pH 7 to 6 for EuCl2(aq) and 2-EuII, respectively, in the reaction with BrO3−. The same reactions with BrO3− resulted in increases in kox of 5.3 and 2.9× from pH 6 to 5 for EuCl2(aq) and 2-EuII, respectively. However, there was no increase in kox from pH 7 to 6 and a 17× increase in kox from pH 6 to 5 for the reaction of 1-EuII with BrO3−. The relatively large increase in kox for 1-EuII upon decreasing from pH 6 to 5 caused a change in the order of oxidation rates of the three Eu-containing species from EuCl2(aq) > 1-EuII > 2-EuII at pH 7 and 6 to 1-EuII > EuCl2(aq) > 2-EuII at pH 5. The 17× increase in kox and the switch in order of oxidation rates demonstrate that 1-EuII had a relatively high sensitivity to change in solution pH.
Figure 3.
Normalized absorbance as a function of time at pH 5 (□), 6 (◊), and 7 (○) for 1-EuII in the presence of (A) BrO3− or (B) GSSG, EuCl2(aq) in the presence of (C) BrO3− or (D) GSSG, and 2-EuII in the presence of (E) BrO3− or (F) GSSG. At the beginning of each reaction, all samples contained EuII (1.00 mM), acetate (10.0 mM, pH 5), 2-morpholinoethane-1-sulfonate (10.0 mM, pH 6), 3-morpholinopropane-1-sulfonate (MOPS, 10.0 mM, pH 7) buffer, and oxidant (10.0 mM). Error bars represent the standard error of the mean of three independently prepared samples.
Table 2.
kox (×10−3 min−1) of 1-EuII, EuCl2(aq), and 2-EuII with BrO3− at pH 5, 6, and 7.[a]
| Complex | pH 5 | pH 6 | pH 7 |
|---|---|---|---|
| 1-EuII | 12 ± 1 | 0.7 ± 0.1 | 0.7 ± 0.1 |
| EuCl2(aq) | 9.5 ± 0.6 | 1.8 ± 0.1 | 1.3 ± 0.1 |
| 2-EuII | 2.0 ± 0.1 | 0.7 ± 0.1 | 0.3 ± 0.1 |
The oxidation of EuII followed a first-order decay. Accordingly, oxidation rates were calculated using the integrated first-order rate law. Error values represent the standard error of the mean of three independently prepared samples.
The relatively high sensitivity of kox to pH for 1-EuII can be explained by protons associated with 1-EuII. The exchange rate of amide protons on the macrocyclic tetraglycinate ligand of 1-EuII with trivalent lanthanides is pH-dependent.[23] The exchange rate of amide protons on 1-EuIII has been examined using radiofrequency saturation transfer of amide protons to bulk water protons at pH 5 (extremely slow amide-proton exchange, kex < 127 s−1) and pH 7 (kex = 127 s−1).[24] Therefore, it could be envisioned that hydrogen bonding between amide protons on 1-EuII and oxygen atoms on BrO3− persist to a greater extent at pH 5 than at pH 6 or 7. The water exchange rate of 1-EuIII (kex = 5.8 × 104 s−1)[24] is orders of magnitude faster than amide proton exchange and is insensitive to changes in pH from 5–8.[23,24] Labile water molecules on EuCl2(aq) and the absence of exchangeable protons on the macrocyclic ligand of 2-EuII likely preclude these complexes from participating in comparable hydrogen bonding interactions with BrO3−. The water-exchange rate of EuCl2(aq) (kex = 4.4 × 109 s−1)[25] and a structurally analogous complex to 2-EuII (kex = 8.5 × 107 s−1)[26] indicate that coordinated water molecules are too fleeting to participate in a similar hydrogen bonding interaction as expected for amide protons. Coordinated water molecules on EuCl2(aq) and 2-EuII might contribute to the observed change in kox, but the relatively large change in kox for 1-EuII is likely due to proton exchange occurring on the macrocyclic ligand rather than labile monodentate water molecules. Accordingly, the relatively slow exchange of amide protons on 1-EuII at pH 5 could allow these protons to hydrogen bond with BrO3− in the vicinity of EuII, thereby increasing the probability of electron transfer. Upon neutralization to pH 7, the hydrogen-bonding interactions are interrupted by relatively fast exchange of amide protons on 1-EuII, thereby decreasing the probability of electron transfer. Protonation of a carboxylate group on 1-EuII could also explain the relatively large increase in kox at pH 5. Protonation would lower the overall charge of the complex and could result in a hydrogen-bonding interaction with BrO3−. Protonation studies of similar tetraglycinate complexes have been reported.[27]
The order of oxidation rates EuCl2(aq) > 2-EuII was observed at pH 7, 6, and 5. This observation can be explained by the cryptand of 2-EuII inhibiting access to the metal center by decreasing available coordination sites from 9 or 10 in EuCl2(aq) to 1 or 2 in 2-EuII.[5] A similar explanation was used to rationalize the decreased rate of EuII oxidation by hydrogen peroxide upon chelation in crown ethers and cryptands.[13] In that study, the decreased oxidation rate was attributed to the steric protection of EuII ions within appropriately sized ligands. By the same logic, EuCl2(aq) would be expected to have more available coordination sites than 1-EuII, indicating that the order of oxidation rates should be EuCl2(aq) > 1-EuII at pH 7, 6, and 5. EuCl2(aq) was oxidized the fastest at pH 7 and 6, but the order of oxidation rates switched to 1-EuII > EuCl2(aq) at pH 5. Therefore, the ability of BrO3− to access open coordination sites on EuII cannot explain these data without another factor governing oxidation rate. A likely factor is the hydrogen-bonding interaction between 1-EuII and BrO3− described above.
Unlike BrO3−, GSSG did not react with 1-EuII, EuCl2(aq), or 2-EuII during the course of the 240 min experiment at pH 5 or 7. The negative Gibbs free energies of electron transfer between GSSG and 1-EuII or EuCl2(aq) indicate that spontaneous reactions should occur between GSSG and these two complexes. The lack of reactivity observed in our experiments indicates the presence of an activation barrier, possibly from the inability of the disulfide bond to access the EuII metal center, preventing disulfide reduction over a 240 min period. Unlike 1-EuII and EuCl2(aq), the lack of reactivity between 2-EuII and GSSG can be explained by positive Gibbs free energies at pH 5 and 7 (Table 1), indicating that spontaneous reactions should not be observed. Altogether, the GSSG data and in vivo imaging experiments[5,6] indicate that, from a biological standpoint, bulky disulfide bonds or other bulky oxidizing agents are likely minor contributors at most to EuII oxidation in vivo for imaging experiments shorter than four hours.
Conclusions
Our findings provide insight into the oxidation chemistry of EuII-containing complexes, such as the formation of O2−, the role of the ligand in EuII oxidation rate, and reaction barriers with disulfide bonds. We expect that the ability to rationally design EuII-containing complexes to tune both thermodynamic stability and kinetics of oxidation will be invaluable in the design of new oxidation-responsive complexes for MRI.
Experimental Section
General remarks
Commercially available chemicals were of reagent-grade purity or better and were used without further purification unless otherwise noted. The EPR spin trap BMPO was purchased from Applied Bioanalytical Labs. Water was purified using a PURELAB Ultra Mk2 water purification system (ELGA). Water and methanol were degassed under reduced pressure prior to use. Oxidation rates were obtained from the slope of natural logarithm of absorbance as a function of time.
Inductively coupled plasma mass spectrometry (ICP–MS)
Measurements of Eu concentrations were acquired on an Agilent Technologies 7700 series ICP–MS instrument at the Lumigen Instrument Center in the Department of Chemistry at Wayne State University. All dilutions were performed with aqueous 2% HNO3, which was also used for blank samples during calibration. Calibration curves were created using the 153Eu isotope ion count for a 1–200 ppb concentration range (diluted from Fluka ICP standard solution, Eu2O3 in aqueous 2% HNO3, 1000 mg Eu/L). All samples were diluted to fall within this range.
EPR spectroscopy
EPR spectroscopy was performed with a Bruker EMX X-band spectrometer equipped with an Oxford variable-temperature cryostat. Acquisition parameters included a temperature of 110 K, microwave frequency of 9.3696 GHz, microwave power of 1.99 mW, modulation amplitude of 1.0 G (with 10 points per modulation amplitude). EPR samples were prepared under an inert atmosphere of Ar within a glovebox, were a total volume of 0.3 mL, and were sealed with wax under an atmosphere of Ar in Norell SEPR250S EPR tubes. Solutions were prepared by dissolving EuCl2 (1.7 mg, 7.6 µmol) and BMPO (1.1 mg, 5.0 µmol) in methanol (0.450 mL). After measuring the sample under an Ar atmosphere, O2 was bubbled directly into the sample solution within the EPR tube for 15 s. The sample was measured 5 min after bubbling O2. The positive control with KO2 was prepared by adding a methanolic solution (0.5 mL) containing BMPO (12 mM) to solid KO2 (0.4 mg, 6 µmol). The control with a direct source of EuIII was prepared in the same manner as the positive control with KO2, but with the addition of EuCl3·6H2O (2.8 mg, 7.6 µmol). The resulting mixtures were stirred vigorously for 15 s before filtering through a 0.2 µm hydrophilic filter into EPR tubes. The resulting samples were measured 5 min after exposure to KO2. The same procedure was used for the sample containing KO2 without BMPO.
Preparation of buffer and oxidant solutions for UV–visible spectroscopy
Buffer for pH 5 reactions was prepared by dissolving sodium acetate trihydrate (68.0 mg, 0.500 mmol) in water (9.00 mL). The solution pH was adjusted to 5 with the addition of aqueous HCl (0.1 M). Additional water was used to obtain a final volume of 10.0 mL. Buffer for pH 6 reactions was prepared by dissolving 2-morpholinoethane-1-sulfonic acid (211.4 mg, 1.083 mmol) in water (10.0 mL). The solution pH was adjusted to 6 with the addition of aqueous NaOH (0.1 M). Additional water was used to obtain a final volume of 20.0 mL. Buffer for pH 7 reactions was prepared by dissolving 3-morpholinopropane-1-sulfonic acid (104.6 mg, 0.500 mmol) in water (9.00 mL). The solution pH was adjusted to 7 with the addition of aqueous NaOH (0.1 M). Additional water was added to obtain a final volume of 10.0 mL. An aqueous stock solution of BrO3− was prepared by dissolving potassium bromate (83.5 mg, 0.500 mmol) in water (4.50 mL). The pH was either left neutral for pH 7 reactions or adjusted to pH 5 or 6 with the addition of aqueous HCl (0.1 M). Additional water was used to reach a final volume of 5.00 mL. An aqueous stock solution of GSSG was prepared by dissolving L-glutathione oxidized (306.3 mg, 0.500 mmol) in water (4.50 mL). The pH was increased to 5 or 7 with the addition of aqueous NaOH (0.1 M). Additional water was used to obtain a final volume of 5.00 mL.
Preparation of tetraglycinate complex for UV–visible spectroscopy (1-EuII)
The preparation of 1-EuII was adapted from a reported procedure.[7] Briefly, 1-EuIII·9H2O (2.00 mg, 2.12 µmol, Macrocyclics) was reduced with Zn dust (30 mg, 0.46 mmol) in water (1.00 mL) followed by filtration through a 0.2 µm hydrophilic filter. DOWEX cation-exchange resin was used to exchange Zn2+ with Na+, and resin was removed by filtration through a 0.2 µm hydrophilic filter. To the resulting solution containing 1-EuII was added water (400 µL), aqueous buffer (400 µL of a 50.0 mM solution), and aqueous oxidant (200 µL of a 100 mM solution) to yield the final reaction solution [2.00 mL of 1-EuII (1.00 mM), buffer (10.0 mM), and oxidant (10.0 mM)].
Preparation of aqua complex for UV–visible spectroscopy (EuCl2(aq))
To water (1303 µL) was added aqueous EuCl2 (97.1 µL of a 20.6 mM solution), aqueous buffer (400 µL of a 50.0 mM solution), and aqueous oxidant (200 µL of a 100 mM solution) to yield the final reaction solution [2.00 mL of EuCl2(aq) (1.00 mM), buffer (10.0 mM), and oxidant (10.0 mM)].
Preparation of 4-fluorobenzo-functionalized complex for UV–visible spectroscopy (2-EuII)
The synthesis and characterization of the 4-fluorobenzo-functionalized ligand is described elsewhere.[5] To water (1259 µL) was added aqueous EuCl2 (97.1 µL of a 20.6 mM solution) and aqueous 4-fluorobenzo-functionalized ligand (44.0 µL of a 50.0 mM solution). The resulting solution was stirred at ambient temperature under an inert atmosphere for 2 h before the addition of aqueous buffer (400 µL of a 50.0 mM solution) and aqueous oxidant (200 µL of a 100 mM solution) to yield the final reaction solution [2.00 mL of 2-EuII (1.00 mM), buffer (10.0 mM), and oxidant (10.0 mM)].
UV–visible data acquisition
Studies were performed under an atmosphere of N2 in a wet glovebox (water allowed, O2 excluded) using an Ocean Optics STS microspectrometer (STS-UV-L-25-400-SMA) coupled to an Ocean Optics high power DH-MINI deuterium tungsten halogen source with shutter (200–2000 nm). A cuvette holder (CUV-UV) equipped with a cover (CUV-COVER) was used to measure samples within the glovebox over a 4 h period. Optical fibers (solarization-resistant, 400 µm optical fibers 1 m in length) from the light source and spectrometer (outside of the glovebox) were connected to both ends of the cuvette holder (inside the glovebox) using a dual VFT, KF-40 flange adapter (2–450 µm XSR) and with a total of 4 × 1 m of optical fiber. Samples were housed in individual air-tight quartz cuvettes and remained capped between measurements. Caps were removed from cuvettes during the measurements because the cuvette cover used to block ambient light would not accommodate the cap. Final reaction solutions (described above) were prepared within the cuvette and were stirred for 15 s prior to the initial measurement, but were not stirred between subsequent measurements. The integration time used for all measurements was 10,000 ms.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (R01EB013663), and L. A. E. is grateful to Wayne State University for a Summer Dissertation Fellowship.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- 1.a) Meihaus KR, Fieser ME, Corbey JF, Evans WJ, Long JR. J. Am. Chem. Soc. 2015;137:9855–9860. doi: 10.1021/jacs.5b03710. [DOI] [PubMed] [Google Scholar]; b) Blagg RJ, Ungur L, Tuna F, Speak J, Comar P, Collison D, Wernsdorfer W, McInnes EJL, Chibotaru LF, Winpenny REP. Nat. Chem. 2013;5:673–678. doi: 10.1038/nchem.1707. [DOI] [PubMed] [Google Scholar]
- 2.a) Kuda-Wedagedara ANW, Wang C, Martin PD, Allen MJ. J. Am. Chem. Soc. 2015;137:4960–4963. doi: 10.1021/jacs.5b02506. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kelly RP, Bell TDM, Cox RP, Daniels DP, Deacon GB, Jaroschik F, Junk PC, Le Goff XF, Lemercier G, Martinez A, Wang J, Werner D. Organometallics. 2015;34:5624–5636. [Google Scholar]; c) de Bettencourt-Dias A, Barber PS, Bauer S. J. Am. Chem. Soc. 2012;134:6987–6994. doi: 10.1021/ja209572m. [DOI] [PubMed] [Google Scholar]
- 3.a) Yin H, Carroll PJ, Anna JM, Schelter EJ. J. Am. Chem. Soc. 2015;137:9234–9237. doi: 10.1021/jacs.5b05411. [DOI] [PubMed] [Google Scholar]; b) Chciuk TV, Anderson WR, Jr, Flowers RA., II Angew. Chem. 2016;128:6137–6140. doi: 10.1002/anie.201601474. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2016;55:6033–6036. doi: 10.1002/anie.201601474. [DOI] [PubMed] [Google Scholar]
- 4.a) Tsitovich PB, Burns PJ, McKay AM, Morrow JR. J. Inorg. Biochem. 2014;133:143–154. doi: 10.1016/j.jinorgbio.2014.01.016. [DOI] [PubMed] [Google Scholar]; b) Ekanger LA, Ali MM, Allen MJ. Chem. Commun. 2014;50:14835–14838. doi: 10.1039/c4cc07027e. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Ratnakar SJ, Soesbe TC, Lumata LL, Do QN, Viswanathan S, Lin C-Y, Sherry AD, Kovacs Z. J. Am. Chem. Soc. 2013;135:14904–14907. doi: 10.1021/ja406738y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ekanger LA, Polin LA, Shen Y, Haacke EM, Martin PD, Allen MJ. Angew. Chem. 2015;127:14606–14609. doi: 10.1002/anie.201507227. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2015;54:14398–14401. doi: 10.1002/anie.201507227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ekanger LA, Polin LA, Shen Y, Haacke EM, Allen MJ. Contrast Media Mol. Imaging. 2016;11:299–303. doi: 10.1002/cmmi.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ekanger LA, Mills DR, Ali MM, Polin LA, Shen Y, Haacke EM, Allen MJ. Inorg. Chem. 2016;55:9981–9988. doi: 10.1021/acs.inorgchem.6b00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekanger LA, Allen MJ. Metallomics. 2015;7:405–421. doi: 10.1039/c4mt00289j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meier DJ, Garner CS. J. Phys. Chem. 1952;56:853–857. [Google Scholar]
- 10.Balzani V, Scandola F, Orlandi G, Sabbatini N, Indelli MT. J. Am. Chem. Soc. 1981;103:3370–3378. [Google Scholar]
- 11.Yee EL, Hupp JT, Weaver MJ. Inorg. Chem. 1983;22:3465–3470. [Google Scholar]
- 12.a) Candlin JP, Halpern J, Trimm DL. J. Am. Chem. Soc. 1964;86:1019–1022. [Google Scholar]; b) Adin A, Sykes AG. Nature. 1966;209:804. [Google Scholar]; c) Adin A, Sykes AG. J. Chem. Soc. A. 1966:1230–1236. [Google Scholar]; d) Carlyle DW, Espenson JH. J. Am. Chem. Soc. 1968;90:2272–2278. [Google Scholar]; e) Chou M, Creutz C, Sutin N. J. Am. Chem. Soc. 1977;99:5615–5623. [Google Scholar]; f) Muralidharan S, Espenson JH. Inorg. Chem. 1984;23:636–639. [Google Scholar]
- 13.Staninski K, Kaczmarek M, Schroeder G, Elbanowski M. Monatsh. Chem. 1999;130:1311–1318. [Google Scholar]
- 14.Zhao H, Joseph J, Zhang H, Karoui H, Kalyanaraman B. Free Radic. Biol. Med. 2001;31:599–606. doi: 10.1016/s0891-5849(01)00619-0. [DOI] [PubMed] [Google Scholar]
- 15.Dvoranová D, Barbieriková Z, Brezová V. Molecules. 2014;19:17279–17304. doi: 10.3390/molecules191117279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.You H, Nogami M. J. Phys. Chem. B. 2004;108:12003–12008. [Google Scholar]
- 17.Gamage N-DH, Mei Y, Garcia J, Allen MJ. Angew. Chem. 2010;122:9107–9109. doi: 10.1002/anie.201002789. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2010;49:8923–8925. doi: 10.1002/anie.201002789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding L, Li Q, Cui H, Tang R, Xu H, Xie X, Zhai J. Electrochim. Acta. 2010;55:8471–8475. [Google Scholar]
- 19.Feig AL, Lippard SJ. Chem. Rev. 1994;94:759–805. [Google Scholar]
- 20.Schafer FQ, Buettner GR. Free Radic. Biol. Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 21.Gerweck LE, Seetharaman K. Cancer Res. 1996;56:1194–1198. [PubMed] [Google Scholar]
- 22.Bousquet J-C, Saini S, Stark DD, Hahn PF, Nigam M, Wittenberg J, Ferrucci JT., Jr Radiology. 1988;166:693–698. doi: 10.1148/radiology.166.3.3340763. [DOI] [PubMed] [Google Scholar]
- 23.a) Aime S, Barge A, Castelli DD, Fedeli F, Mortillaro A, Nielsen FU, Terreno E. Magn. Reson. Med. 2002;47:639–648. doi: 10.1002/mrm.10106. [DOI] [PubMed] [Google Scholar]; b) Aime S, Castelli DD, Terreno E. Angew. Chem. 2002;114:4510–4512. [Google Scholar]; Angew. Chem. Int. Ed. 2002;41:4334–4336. doi: 10.1002/1521-3773(20021115)41:22<4334::AID-ANIE4334>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 24.Terreno E, Castelli DD, Cravotto G, Milone L, Aime S. Invest. Radiol. 2004;39:235–243. doi: 10.1097/01.rli.0000116607.26372.d0. [DOI] [PubMed] [Google Scholar]
- 25.Caravan P, Tóth É, Rockenbauer A, Merbach AE. J. Am. Chem. Soc. 1999;121:10403–10409. [Google Scholar]
- 26.Garcia J, Neelavalli J, Haacke EM, Allen MJ. Chem. Commun. 2011;47:12858–12860. doi: 10.1039/c1cc15219j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baranyai Z, Brücher E, Iványi T, Király R, Lázár I, Zékány L. Helv. Chim. Acta. 2005;88:604–617. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.