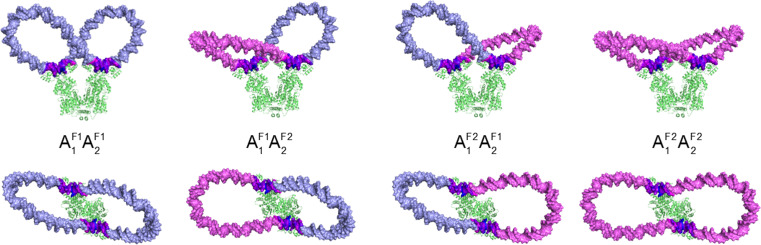
Fig. 3.
Molecular images showing the overall folding and torsional stress in a 182-bp DNA minicircle found upon attaching a pair of evenly spaced operators in all possible antiparallel orientations to the Lac repressor. Configurations are denoted by the settings and families of the energy-optimized loops anchored to the protein (see text). Views looking perpendicular to (upper row) and down (lower row) the symmetry axis of the V-shaped protein assembly. The torsional stress is expressed in terms of the net change in the twist of supercoiling, relative to B DNA, at individual base-pair steps along the closed molecule. DNA is color-coded such that the most underwound steps (located on the operators) are depicted in deep blue and the most overwound steps (also on the operators) in deep magenta, with intermediate regions of negative, null, and positive deviations in twist varying respectively from blue to white to magenta. The uniform twist along the protein-free lobes reflects the treatment of DNA as an ideal elastic rod