Fig. 4.
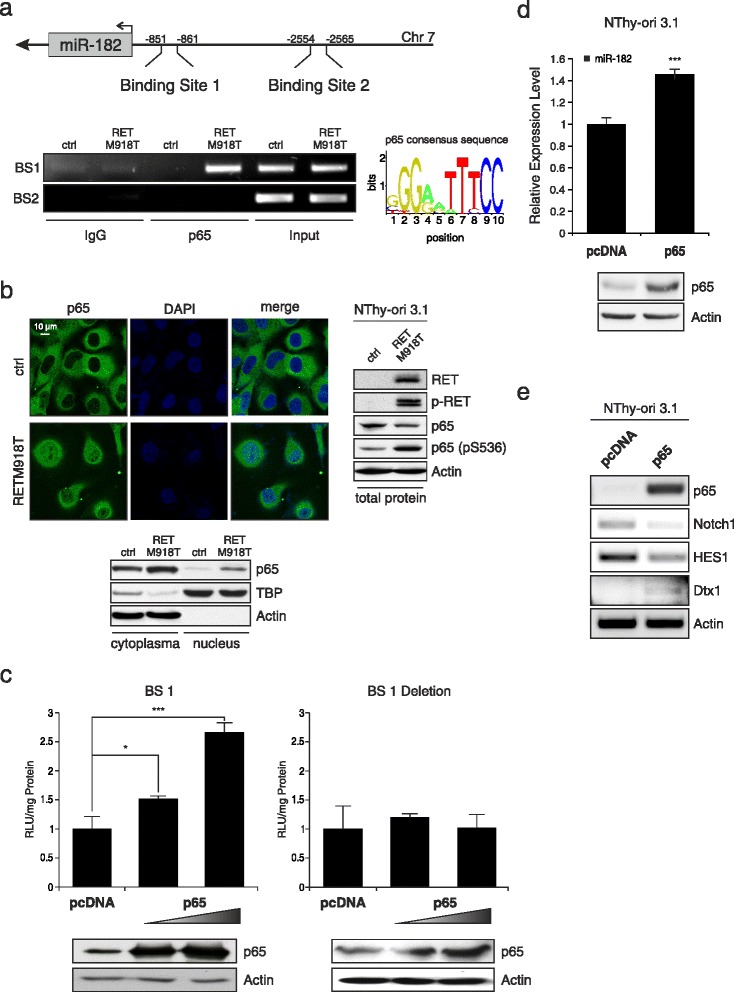
Oncogenic RET-activated NF-κB induces miR-182 expression. a Schematic diagram of the promoter region with two putative p65 binding motifs at positions −851 (binding site 1, BS1) and −2554 (binding site 2, BS2) upstream of the transcriptional start of miR-182 (top). The graph shows the consensus sequence used for identification of p65 binding sites. ChIP assays were performed using anti-NF-κB p65 and IgG as negative control (bottom). b Localization and expression of NF-κB p65 in thyroid cells stably expressing mutant RETM918T analyzed by immunofluorescence and Western blot. Blots were probed with RET, p-RET, NF-κB p65, NF-κB p65 (pS536), and Actin, or TBP for equal loading of the cytoplasmic and nuclear fraction, respectively. Western blot on the right represents total protein lysate. DAPI was used for nuclear staining. c Luciferase assays of NThy-ori 3.1 cells cotransfected with 1 μg miR-182 promoter (left) or BS1 deletion construct (right) and increasing amounts (1.0, 2.0 μg) of p65 expression plasmid or pcDNA (1 μg) as control. Reporter activities are shown as fold activation relative to the control after normalization to total protein concentration in cell extracts at 24 h post transfection. Bar graphs are means ± S.D; * p < 0.05, *** p < 0.001. Expression levels of NF-κB p65 and actin as loading control are indicated by Western blot. d Expression of miR-182 in NThy-ori 3.1 cells transfected with p65 or pcDNA was determined by TaqMan qRT-PCR. For relative expression level empty pcDNA was set as one; *** p < 0.001. e Effect of ectopic NF-κB p65 expression in normal thyroid cells on Notch1, HES1, and Dtx1 RNA levels. Actin served as loading control
