Fig. 1.
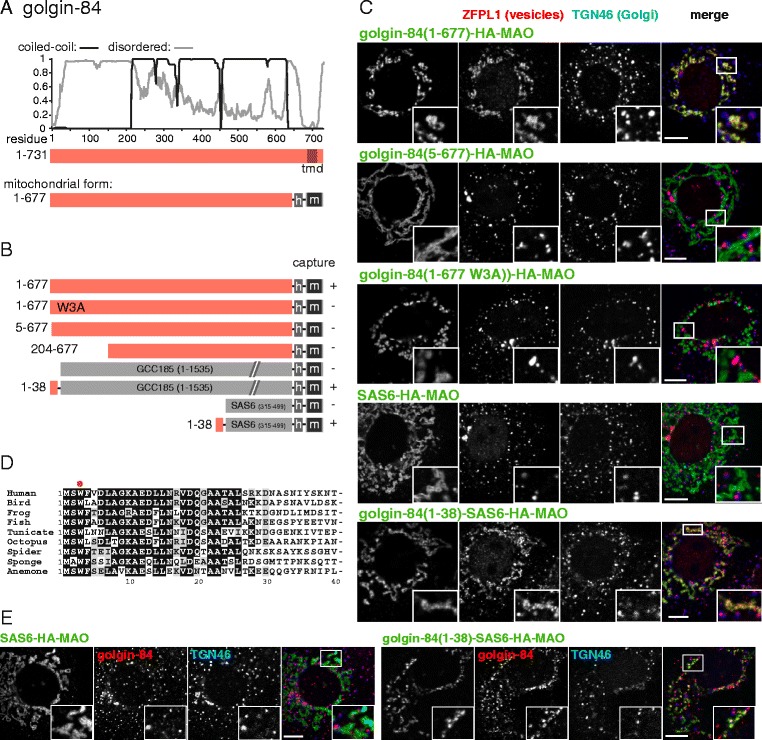
Mapping the part of golgin-84 that can capture vesicles. a Schematic diagram of human golgin-84 along with plots for the predicted degree of coiled-coil and disorder along its length. Also shown is the mitochondrial form in which the Golgi-targeting transmembrane domain (TMD) is replaced with a hemagglutinin (HA) tag (h) and the TMD of human monoamine oxidase A (m), a protein of the outer mitochondrial membrane. b Summary of the vesicle capture activity of the indicated variants of mitochondrial golgin-84. Capture at mitochondria was assayed by immunofluorescent staining of the Golgi integral membrane proteins ZFPL1, giantin and GalNAc-T2. Plus sign indicates that capture of all three markers was similar to the wild-type protein, minus sign indicates that no significant capture was observed. c Confocal micrographs of HeLa cells expressing the indicated golgin-84 variants and stained for the HA tag on the golgin-84 chimera as well as for ZFPL1 (in vesicles captured by golgin-84), and for TGN46 (a Golgi protein that is not captured). Cells were treated with nocodazole for 6 h prior to fixation to ensure that mitochondria were close to intra-Golgi transport vesicles. Key constructs from the set shown in (b) are included, with similar results obtained using the markers giantin and GalNAc-T2. Scale bars 10 μm. d Alignment of the N-terminus of human golgin-84 with that from the indicated species. Bird, G. gallus; frog, X. laevis; fish, T. rubripes; tunicate, C. savignyi; octopus, O. bimaculoides; spider, S. mimosarum; sponge, A. queenslandica; anemone, N. vectinis. Well conserved residues are shaded. e As c except that the cells were stained for golgin-84 using an antibody that binds outside of residues 1–38. This indicates that the N-terminus of golgin-84 is sufficient to capture vesicles containing golgin-84. Scale bars 10 μm
