Abstract
Background
Clinical studies have demonstrated that the early and empiric use of plasma improves survival after hemorrhagic shock. We have demonstrated in rodent models of hemorrhagic shock that resuscitation with plasma is protective to the lungs compared to lactated Ringers. As our long term objective is to determine the molecular mechanisms that modulate plasma’s protective effects in injured bleeding patients, we have used human plasma in a mouse model of hemorrhagic shock. The goal of the current experiments is to determine if there are significant adverse effects on lung injury when using human versus mouse plasma in an established murine model of hemorrhagic shock and laparotomy.
Methods
Mice underwent laparotomy and 90 minutes of hemorrhagic shock to a mean arterial pressure of 35±5 mm Hg followed by resuscitation at 1× shed blood using either mouse fresh frozen plasma (FFP), human fresh frozen plasma or human lyophilized plasma. Mean arterial pressure (MAP) was recorded during shock and for the first 30 minutes of resuscitation. After 3 hours, animals were euthanized and lungs collected for analysis.
Results
There was a significant increase in early MAP when mouse FFP was used to resuscitate animals compared to human FFP or human lyophilized plasma. However in spite of these differences, analysis of the mouse lungs revealed no significant differences in pulmonary histopathology, lung permeability or lung edema between all three plasma groups. Analysis of neutrophil infiltration in the lungs revealed that mouse FFP decreased neutrophil influx as measured by neutrophil staining, however myeloperoxidase immunostaining revealed no significant differences in between groups.
Conclusions
The study of human plasma in a mouse model of hemorrhagic shock is feasible, but does reveal some differences compared to mouse plasma-based resuscitation in physiologic measures such as MAPs post-resuscitation. Measures of end organ function such as lung injury appear to be comparable in this acute model of hemorrhagic shock and resuscitation.
Keywords: hemorrhagic shock in mice, lyophilized plasma, transfusion reaction
INTRODUCTION
Hemorrhage remains the leading cause of early deaths in severely injured patients and combat casualities (1,2). Current efforts are focusing on optimizing the timing and ratios of blood component administration (3). The early and empiric use of fresh frozen plasma (FFP) in hemodynamically unstable patients and combat casualties with bleeding has led to a decrease in early hemorrhagic deaths (4–6), with some centers now employing plasma in the pre-hospital setting (7,8). Over 335,000 units of blood products were transfused in Iraq and Afghanistan as of 2015 with approximately one-third of the units being fresh-frozen plasma (FFP) (9). The decrease in mortality from a plasma-based resuscitation strategy appears to extend beyond its ability to correct trauma-induced coagulopathy or volume expansion and is believed to involve correction of the inflammatory processes and the endothelial injury that follows trauma and hemorrhage (10). The endotheliopathy of trauma (EOT) leads not only to coagulation abnormalities but also to inflammation, breakdown of endothelial barrier integrity, tissue edema and end organ injury. (11)
We have shown in vitro and in vivo using rodent models of hemorrhagic shock (HS) that resuscitation with plasma versus lactated ringers provides significant benefits to the pulmonary vasculature by reducing injury, inflammation, and permeability. In vitro studies using human endothlelial cell lines demonstrated that human FFP compared to lactated Ringers (LR) reduced permeability, inflammation and restored vascular barrier compromise induced by VEGF-A and hypoxia. (12–14) In a rat model of hemorrhagic shock using rat plasma or lactated Ringers, rat plasma resulted in lower volume requirements and reduced lung histopathologic injury. (10) Using a similar murine hemorrhagic shock model with human fresh frozen plasma (hFFP) or lactated Ringers resuscitation, hFFP lessened pulmonary hyperpermeability and inflammation as well as gut injury and inflammation compared to lactated Ringers. (15–16) Importantly, this study of human plasma in mice allowed us to test the effects of the clinical product used in humans. We believe this to be of translational benefit and therefore the goal of the current study was to compare, in the same murine model, the effects of resuscitation with human vs. mouse plasma on lung injury induced by HS and laparotomy.
MATERIALS AND METHODS
Mouse model of hemorrhagic shock
All animal procedures performed were approved by the University of Texas Houston Medical School. The experiments were conducted in compliance with the National Institutes of Health guidelines on the use of laboratory animals. All animals were housed at constant room temperature with a 12:12-h light-dark cycle with access to food and water ad libitum. Male C57BL/6J mice 8–10 weeks of age and weighing approximately 20 grams, were used for all experiments. An established coagulopathic mouse model of trauma-hemorrhagic shock was utilized. (16) Under isoflurane anesthesia, a midline laparotomy incision was made, the organs inspected, then the incision closed. Bilateral femoral arteries were cannulated for continuous hemodynamic monitoring and blood withdrawal or resuscitation. After a 10-minute period of equilibration, mice were bled to a mean arterial pressure (MAP) of 35±5 mmHg and maintained for 90 minutes by additional withdrawal of blood or infusion of lactated Ringers and adjustment of the isoflurane. Mice were resuscitated with either mouse FFP (mFFP), human FFP (hFFP), or human lyophilized plasma (hLP) at 1× shed blood. Thirty minutes after resuscitation, vascular catheters were removed, incisions closed, and the animals were awoken from anesthesia. Three hours after the completion of shock, animals were sacrificed by exsanguination under isoflurane anesthesia and lungs harvested for analysis. Human single donor FFP was obtained from Gulf Coast Regional Blood Bank, Houston, TX; donor ABO unknown. Plasma was thawed at 37°C, aliquoted, then refrozen at −80°C until the day of use. Single donor human lyophilized plasma was obtained from HEMCON in Portland, Oregon, and reconstituted the day of use in a citrate diluted in water. Mouse fresh frozen plasma was obtained from a separate set of mice. Blood was obtained via cardiac puncture under isoflurane anesthesia, centrifuged at 500 g for 10 minutes at 4°C, pooled, aliquoted, then frozen at −80°C until the day of use at which time it was thawed at 37°C.
Hemodynamic Data
Mean arterial blood pressure was recorded via the femoral arterial line at baseline then every 5 minutes during the shock period and for the first 30 minutes of resuscitation. After 30 minutes, the lines were removed and the animals awoken to more closely mimic an awake shock model.
Lung Analyses
Histopathology
Lung tissue sections were stained with hematoxylin and eosin (H&E) and scored on a 3-point scale for alveolar thickness, capillary congestion, and cellularity as described by Hart et al. (17) The overall lung injury score was calculated by averaging the parameters.
Inflammation
For neutrophil infiltration, lung tissue sections were mounted on slides and neutrophils stained using the Naphthol AS-D Chloroacetate Esterase Staining kit (Sigma Diagnostics, Milwaukee, Wis), which identifies specific leukocyte esterases. (18) Tissue was stained and neutrophils counted per area of tissue in a blinded fashion by light microscopy at 100× magnification. Myeloperoxidase (MPO) is also an indicator of neutrophil infiltration. Paraffin-embedded tissue was cut into 5 µm-thick sections then incubated with MPO primary antibody (1:100 MPO mouse monoclonal antibody; Abcam, Cambridge, Mass) followed by incubation with secondary antibody (goat antiYmouse; Alexa Fluor 568; Invitrogen). Two random images were taken from each lung section with a fluorescent microscope (Nikon Eclipse Ti, Melville, NY) at 200× magnification and immunofluorescence quantified using ImageJ software (National Institutes of Health). Results are expressed as relative fluorescent units (RFU).
Permeability
To measure Evans blue dye extravasation, animals received an intravenous injection of 3% Evans blue (4 mL/kg) two hours after the completion of resuscitation. One hour later, at the time of sacrifice, animals were perfused via right ventricle with 4°C PBS for 10 minutes to remove intravascular dye followed by 4% paraformadehyde at 4°C. Lungs were harvested then incubated in N-methylformamide for 24 h at 55°C to allow for dye extraction. After centrifugation, absorbance was measured in the supernatant at 620 nm using the VersaMax plate reader (Molecular Devices Inc, Sunnyvale, Calif).
Edema
Lung tissue was weighed and then dried to constant weight at 50°C for 72 hours. The ratio of wet-to-dry was calculated by dividing the wet weight by the dry weight.
Statistical Analysis
Mean arterial pressure was assessed by two-way ANOVA with Bonferroni correction for multiple comparisons. Lung data is reported as mean ± SEM or median (interquartile range [IQR]) with n=8/group and analyzed by one-way ANOVA with Bonferroni post hoc or Kruskal-Wallis test.
RESULTS
Mean arterial pressure (MAP)
MAP was similar between groups at baseline and during shock. However there was a significant difference in MAP between groups during resuscitation with mFFP restoring pressure superior to that of hFFP or hLP (Figure 1).
Figure 1. Mean arterial blood pressure (MAP).
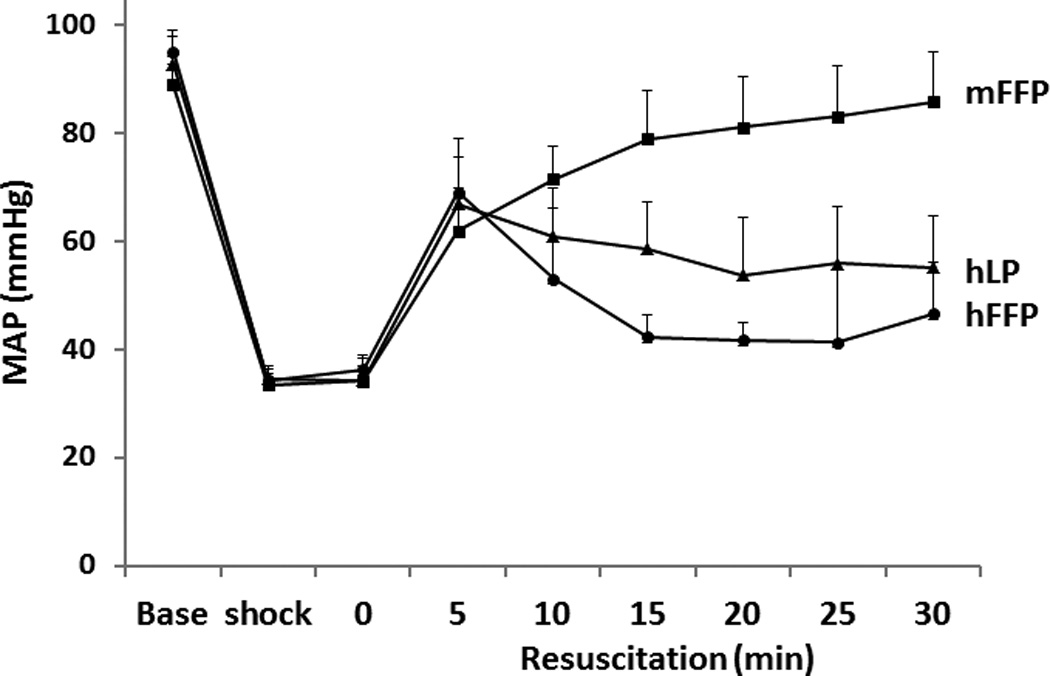
MAP was recorded at baseline then every 5 minutes during the 90 minutes of shock and for the first 30 minutes of resuscitation. MAP was similar between groups at baseline and during shock but during resuscitation, mFFP resulted in a higher MAP. Results were assessed by two-way ANOVA with Bonferroni correction for multiple comparisons, n=8/group. Abbreviations: mFFP=mouse fresh frozen plasma; hLP= human lyophilized plasma; and hFFP= human fresh frozen plasma
Effect of donor plasma species on pulmonary outcomes
When lung histopathologic injury scores were compared, there were no significant differences between groups (Figure 2). Resuscitation with mFFP resulted in a lung injury score of 0.63 ± 0.18, hFFP 0.82 ± 0.01 and hLP 0.90 ± 0.10 (p>0.05).
Figure 2. Lung histopathologic injury was similar between groups.
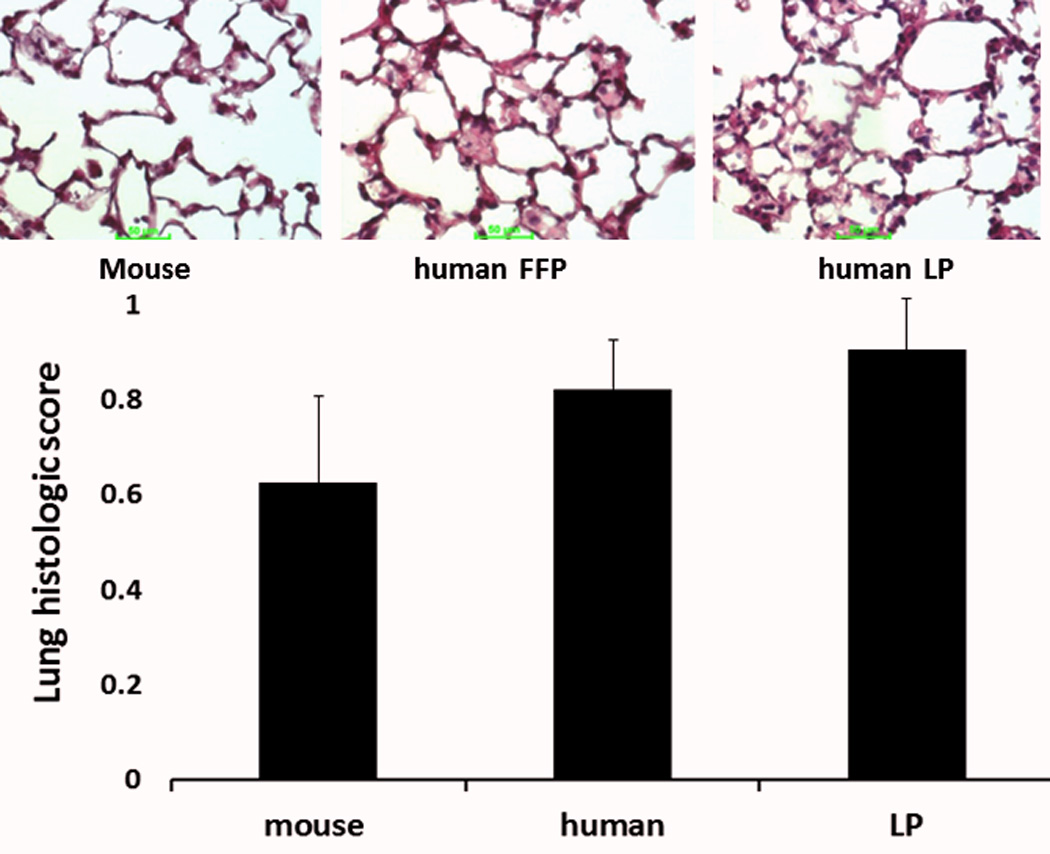
Mice underwent 90 minutes of hemorrhagic shock followed by resuscitation with 1× shed blood of mouse fresh frozen plasma, human fresh frozen plasma, or human lyophilized plasma. Shown are representative images and the corresponding lung injury scores. Data is reported as mean ± SEM with n=8/group and was analyzed by one-way ANOVA with Bonferroni post hoc. Abbreviations: FFP=fresh frozen plasma; LP= lyophilized plasma
Inflammation was quantitated using two different methodologies. Neutrophil infiltration, as measured by Naphthol AS-D Chloroacetate Esterase readings, per area of tissue was significantly less for mFFP (17 ± 1) compared to hFFP (25 ± 2) (p<0.01) and for hLP (19 ± 1) compared to hFFP (p=0.016) (Figure 3A). However, there was no significant difference between mFFP and hLP (p>0.05). In contrast, MPO immunofluorescence, another measure of neutrophil activity, demonstrated no significant differences between groups [mFFP 1,773,706 (2,629,293−1,161,349) vs hFFP 1,880,697 (2,559,481−1,069,840) and hLP 2,571,425 (3,247,373−1,913,976) RFU, p>0.05] as shown in Figure 3B.
Figure 3. Lung inflammation.
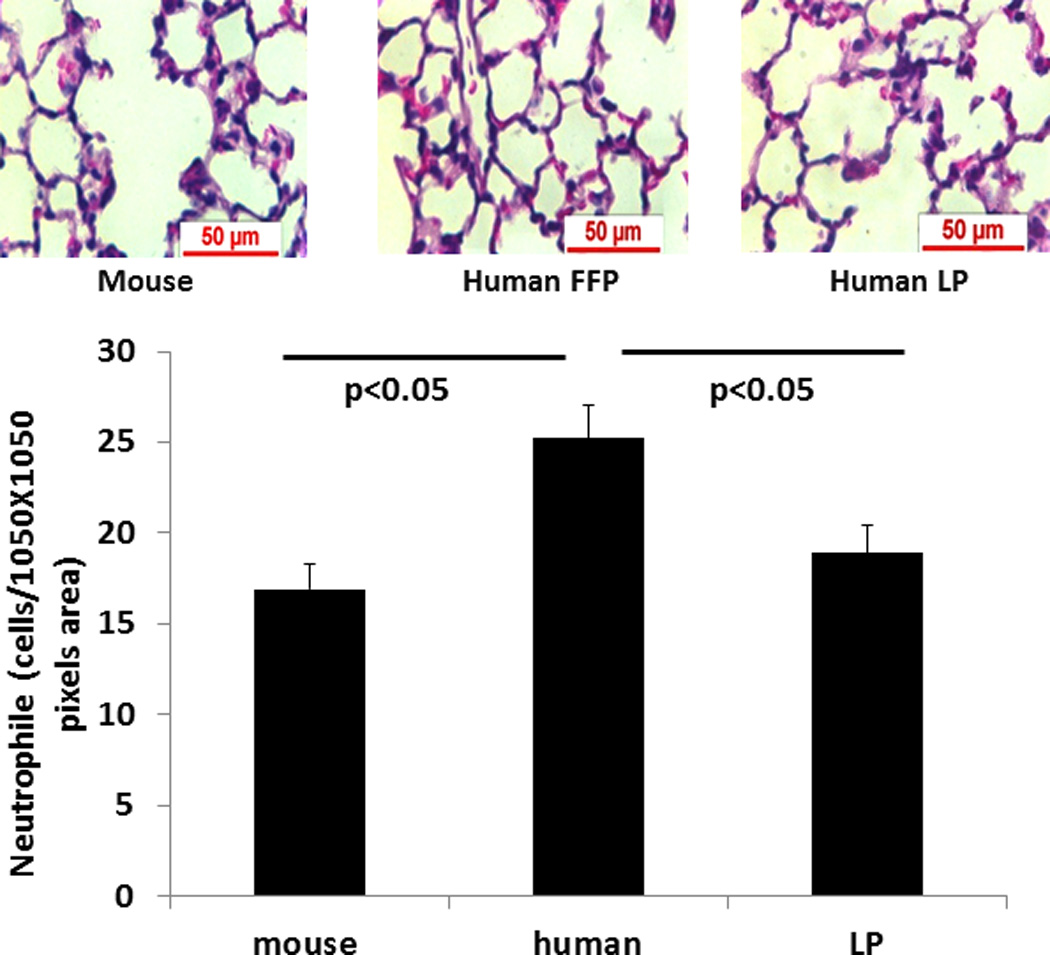
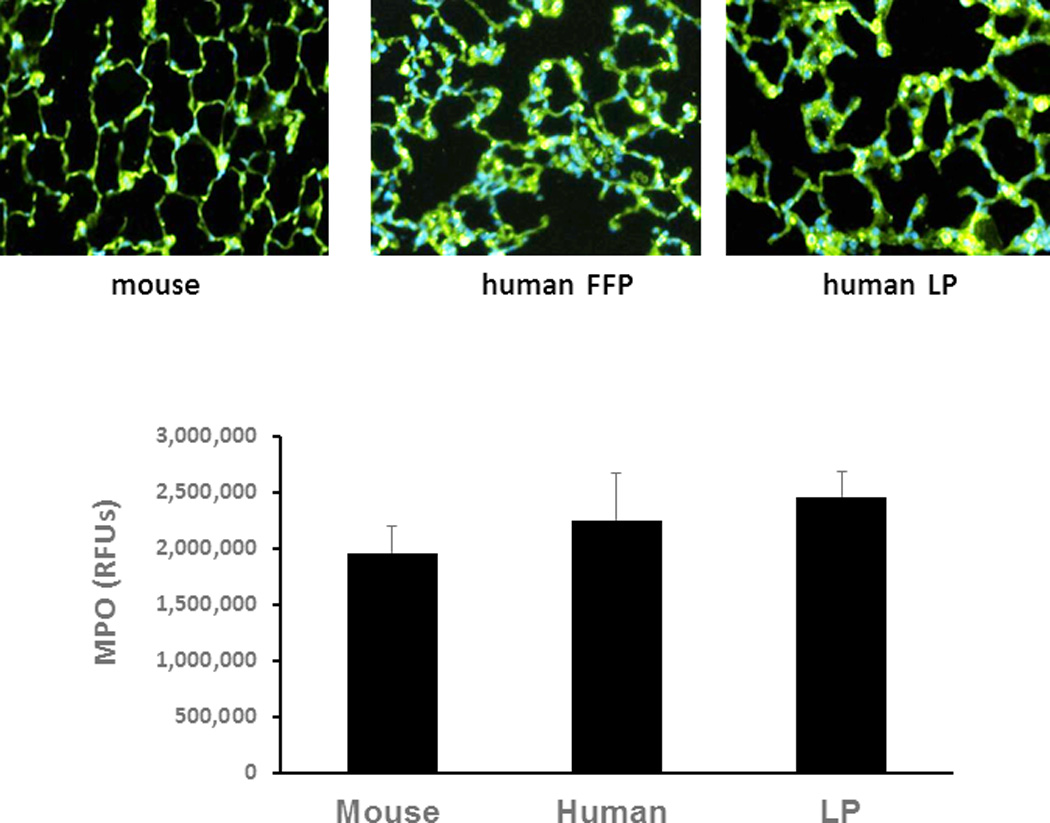
Mice underwent 90 minutes of hemorrhagic shock followed by resuscitation with 1× shed blood of mouse fresh frozen plasma, human fresh frozen plasma, or human lyophilized plasma. In (A) lung neutrophil influx was evaluated. Data is reported as mean ± SEM with n=8/group and was analyzed by one-way ANOVA with Bonferroni post hoc. Myeloperoxidase immunostaining (B) was also assessed. Data is reported as median (interquartile range [IQR]) with n=8/group and analyzed by Kruskal-Wallis test. Shown are representative images and the corresponding quantitation. Abbreviations: FFP=fresh frozen plasma; LP= lyophilized plasma.
Lung permeability assessed by Evan’s Blue dye extravasation was similar between groups with a score (in arbitrary units) of 5.1 ± 0.30 for mFFP, 5.7 ±0.45 for hLP and 6.1 ± 0.49 for hFFP (p>0.05) (Figure 4A). Similarly, there was no significant difference in lung edema between groups (Figure 4B). The lung wet:dry ratio for the mouse plasma group was 3.4 ± 0.2, for hLP 3.1 ± 0.2, and for hFFP 3.2 ± 0.2 (p>0.05).
Figure 4. Lung permeability and edema were similar between groups.
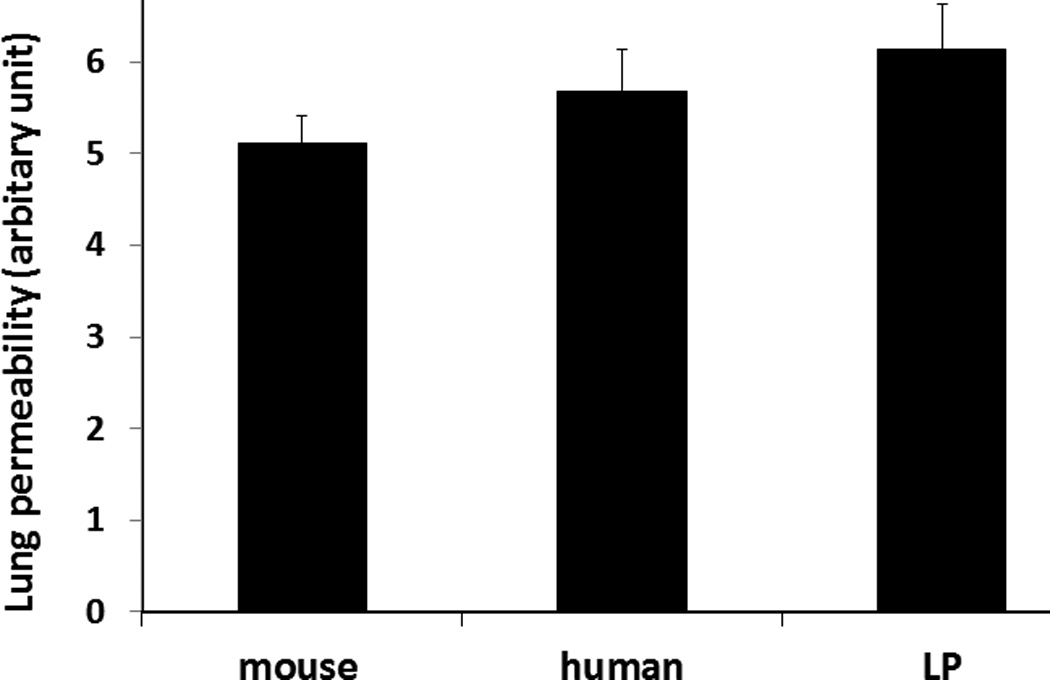
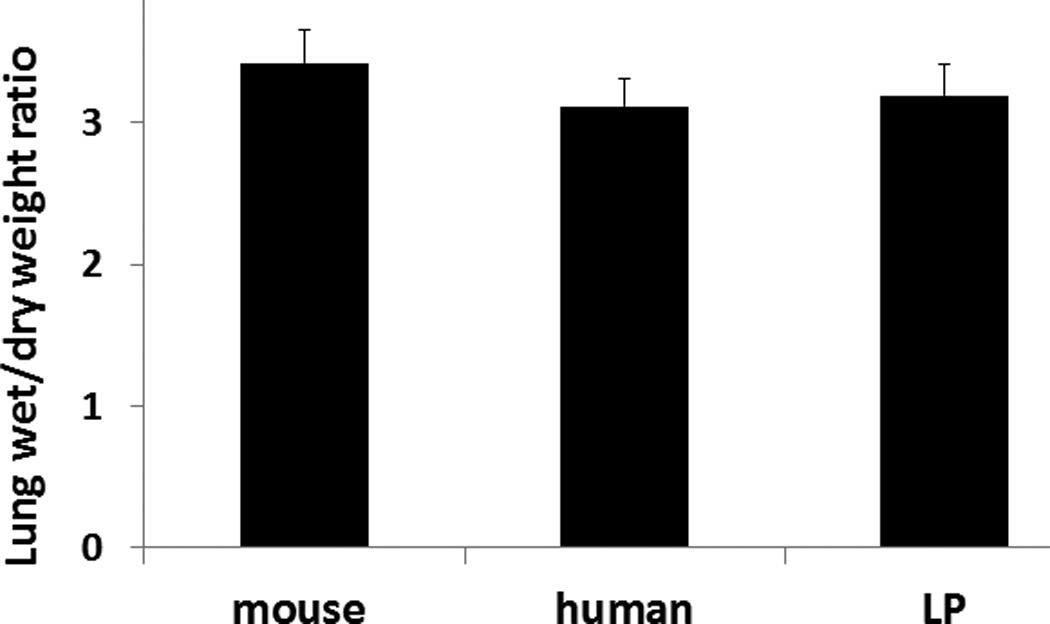
Mice underwent 90 minutes of hemorrhagic shock followed by resuscitation with 1× shed blood of mouse fresh frozen plasma, human fresh frozen plasma, or human lyophilized plasma then lung permeability (A) and edema (B) were measured. Data is reported as mean ± SEM with n=8/group and was analyzed by one-way ANOVA with Bonferroni post hoc. Abbreviations: LP= lyophilized plasma.
DISCUSSION
Comparing mouse to human plasma as a resuscitative agent in a mouse model of hemorrhagic shock, we demonstrated that although there were differences in mean arterial pressure during the early resuscitation phase there were no significant differences in end organ injury as determined by pulmonary histopathology, lung permeability, or lung edema. There was less neutrophil infiltration into the lung when mFFP or hLP was used compared to hFFP, but parameters were similar for myeloperoxidase, another measure of neutrophil infiltration. It is possible that neutrophil influx as determined by Naphthol AS-D Chloroacetate Esterase, is a more sensitive indicator of inflammation than MPO, explaining the differences between inflammatory assays. In the current study we used an immunofluorescent assay to measure MPO quantity rather than MPO activity. We have used both methodologies and not found any significant differences in results. (16)
While xenotransfusions have been researched and performed clinically, to the authors’ knowledge, data specifically related to human plasma transfusion into mice recipients has not been reported (19). We believe it is important to study human plasma in rodent models as there are differences in plasma proteins and we are interested in understanding the mechanisms by which plasma is protective to the human endothelium. Our long range goal would be to specifically identify which protein(s) in plasma contribute to its endothelial protection and then to potentially develop targeted therapy. Also the study of human plasma in mice allows us to test the effects of the clinical product used in humans. All of our reported assays were acute (lasting only 3 hours) and used single donor human plasma. Nevertheless, xeno incompatibility due to the presence pα-gal antibodies in the human plasma and the expression of pα-gal epitope on mouse cells is possible and was not investigated (20). An alternative to using immune-competent mice is to use NOD scid gamma mice (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ as these mice are immunocompromised and can be used to study xenotransplantation with human tissue, cell, and blood products, however the immune response to injury is indeed altered in these mice and will not accurately reflect the molecular and cellular scenarios and cascades taking place after traumatic injury and hemorrhage. This area is worthy of further investigation (21)
One possible reason for some of the noted differences is that the plasma transfusions using human plasma into mice were not matched for ABO compatibility. ABO plasma incompatibility rarely leads to transfusion reactions and precisely how these compare between humans and mice is not well studied. Yamamoto et al cloned murine genomic and complementary DNA and found that the mouse genome contained the human ABO gene equivalent and that the organization of the murine gene was similar to the human counterpart. (22) Functional analysis revealed that the mouse equivalent of the human ABO gene was really an AB gene that encoded proteins that expressed A and B antigens.
One the other hand there is always a risk of transfusion related complications such as transfusion related acute lung injury (TRALI). TRALI can occur with plasma transfusion, which can occur both in the clinical setting as well as in mouse models of transfusion. (23,24) We cannot exclude TRALI as an etiology for the increased neutrophil influx in mice receiving human plasma, but it would be unlikely. While more commonly reported in red blood cell transfusions, hemolytic transfusion reactions can occur in plasma only transfusions. Hemolytic transfusion reactions are presumed to be related to naturally occurring alloantibody in plasma against red blood cell antigen, and can lead to intra- and extravascular hemolysis resulting in hypotension, pigment nephropathy and coagulopathy. It is possible that the lower MAPs achieved with human plasma compared to mouse plasma was due to a hemolytic reaction, though this was not specifically studied. Lastly, transfusion associated circulatory overload (TACO) may occur with large volume transfusions and lead to pulmonary edema, though this would not likely be a contributing factor in the current study.
In addition to studying hFFP, we also examined the effects of hLP on the endothelium. Logistic challenges exist in the use of FFP in the battlefield. In response, dried plasma technologies have been developed to provide a plasma-based resuscitation in the forward environment. (9) Dried plasma is most often lyophilized but can also be spray dried. Lyophilized plasma is not new, it was widely used by British and American forces in WWII and the Korean War. (25) Although it solved the logistical problem its use was stopped because of disease transmission. Modern methods to improve blood safety have made it possible to produce safe and effective dried plasma. Dried plasma products are now available in a number of countries, but to date, not in the United States. Additional benefits to dried products include rapid reconstitution, improved storage profile, and the ability to bank products.
There are a number of limitations of the current study. Only human FFP was frozen and thawed twice (at the time of human donation and then for aliquotting), which may have affected the results. Second, we only recorded MAP during the early phase of resuscitation when mice were still under anesthesia and not over a prolonged period of time that could have revealed more long term or lasting differences. Additionally, we did not specifically observe animals for potential transfusion-related complications. We also did not assess renal functional parameters. It is possible that with longer periods of observation, additional differences may have been demonstrated, including TRALI which may occur up to six hours after transfusion. In prior studies, lungs harvested at 24 hours and five days after resuscitation with hFFP, but not mFFP, and assessed for many of the pulmonary endpoints reported in the current study, demonstrated that lung parameters with plasma resuscitation had returned to sham levels by 24 hours (unpublished data), suggesting that there were no longer term transfusion-related complications. Lastly, a Type II error cannot be excluded so that it is possible additional differences in lung parameters may exist.
In conclusion, we have demonstrated that using human plasma in a mouse model of hemorrhagic shock results in generally comparable findings on lung injury when compared to mouse plasma. Caution should be utilized however, if examining hemodynamic endpoints, as these may differ due to cross species transfusion reactions. Further investigation is warranted to clearly evaluate these differences. We believe our findings support the use of animal models in the evaluation of human blood products for regulatory purposes, in a manner similar to that employed in other drugs and fluids.
Acknowledgments
This work was supported by the National Institutes of Health RO1GM107482 and the Department of Defense W81XWH-11-2-006.
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the US Department of Defense or the US Government
Footnotes
The authors have no conflicts of interest to report
Author contributions: R.A.K. and S.P. conceived and designed this study. Z.P. was responsible for the conduct of the study. Z.P., A.H., and R.A.K. contributed to data analysis. R.A.K. drafted the manuscript and prepared figures. Z.P., R.A.K., S.P., K.H., and M.F. edited and revised the manuscript.
REFERENCES
- 1.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(6 Suppl):S3–S11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 2.Eastridge BJ, Mabry RL, Seguin P, et al. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma Acute Care Surg. 2012;73:S431–S437. doi: 10.1097/TA.0b013e3182755dcc. [DOI] [PubMed] [Google Scholar]
- 3.Cotton BA, Reddy N, Hatch QM, Lefebrvre E, Wade CE, Kozar RA, Gill BS, Albarado R, McNutt MK, Holcomb JB. Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg. 2011;254(4):598–605. doi: 10.1097/SLA.0b013e318230089e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal L, Schreiber MA, Gonzalez EA, Pomper GJ, Perkins JG, Spinella PC, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 5.Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, Alarcon LH, Bai Y, Brasel KJ, Bulger EM, Cotton BA, Matijevic N, Muskat P, Myers JG, Phelan HA, White CE, Zhang J, Rahbar MH PROMMTT Study Group. The prospective, observational, multicenter, massive transfusion study, PROMMTT: comparative effectiveness of a time-varying treatment and competing risks. JAMA Surg. 2013;148(2):127–136. doi: 10.1001/2013.jamasurg.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holcomb JB, Tilley BC, Baraniuk S, Fox EF, Wade CE, Podbielski J, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, Cohen MJ, Cotton BA, Fabian TC, Inaba K, Kerby JD, Muskat P, O'Keeffe T, Rizoli S, Robinson BR, Scalea TM, Schreiber MA, Stein DM, Weinberg JA, Callum JL, Hess JR, Matijevic N, Miller CN, Pittet JF, Hoyt DB, Pearson GD, Leroux B, van Belle G PROPPR Study Group. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma. The PROPPR randomized clinical trial. JAMA. 2015;313(5):471–482. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapman MP, Moore EE, Chin TL, Ghasabyan A, Chandler J, Stringham J, Gonzalez E, Moore HB, Banerjee A, Silliman CC, Sauaia A. Combat: Initial experience with a randomized clinical trial of plasma-based resuscitation in the field for traumatic hemorrhagic shock. Shock. 2015;44(S1):63–70. doi: 10.1097/SHK.0000000000000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holcomb JB, Donathan DP, Cotton BA, Del Junco DJ, Brown G, Wenckstern TV, Podbielski JM, Camp EA, Hobbs R, Bai Y, Brito M, Hartwell E, Red Duke J, Wade CE. Prehospital transfusion of plasma and red blood cells in trauma patients. Prehosp Emerg Care. 2015;19(1):1–9. doi: 10.3109/10903127.2014.923077. [DOI] [PubMed] [Google Scholar]
- 9.Pusateri AE, Given MB, Macdonald VM, Homer MJ. Comprehensive US government program for dried plasma development. Transfusion. 2016;56:S16–S23. doi: 10.1111/trf.13331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozar RA, Peng Z, Zhang R, Holcomb JB, Pati S, Park P, Ko TC, Paredes A. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg. 2011;112:1289–1295. doi: 10.1213/ANE.0b013e318210385c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenkins DH, Rappold JF, Badloe JF, Berséus O, Blackbourne L, Brohi KH, Butler FK, Cap AP, Cohen MJ, Davenport R, Depasquale M, Doughty H, Glassberg E, Hervig T, Hooper TJ, Kozar R, Maegele M, Moore EE, Murdock A, Ness PM, Pati S, Rasmussen T, Sailliol A, Schreiber MA, Sunde GA, van de Watering LM, Ward KR, Weiskopf RB, White NJ, Strandenes G, Spinella PC. THOR Position Paper on Remote Damage Control Resuscitation: Definitions, Current Practice and Knowledge Gaps. Shock. 2014;41(Suppl 1):3–12. doi: 10.1097/SHK.0000000000000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pati S, Matijevic N, Doursout MF, Ko T, Cao Y, Deng X, Kozar RA, Hartwell E, Conyers J, Holcomb JB. Protective effects of fresh frozen plasma on vascular endothelial permeability, coagulation, and resuscitation after hemorrhagic shock are time dependent and diminish between days 0 and 5 after thaw. J Trauma. 2010;69(Suppl 1):S55–S63. doi: 10.1097/TA.0b013e3181e453d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wataha K, Menge T, Deng X, Shah A, Bode A, Holcomb JB, Potter D, Kozar RA, Spinella PC, Pati S. Spray-dried plasma and fresh frozen plasma modulate permeability and inflammation in vitro in vascular endothelial cells. Transfusion. 2013;53(Suppl 1):80S–90S. doi: 10.1111/trf.12040. [DOI] [PubMed] [Google Scholar]
- 14.Potter DR, Baimukanova G, Keating SM, Deng X, Chu JA, Gibb SL, Peng Z, Muench MO, Fomin ME, Spinella PC, Kozar R, Pati S. Fresh frozen plasma and spray-dried plasma mitigate pulmonary vascular permeability and inflammation in hemorrhagic shock. J Trauma Acute Care Surg. 2015;78(6 Suppl 1):S7–S17. doi: 10.1097/TA.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 15.Haywood-Watson RJ, Holcomb JB, Gonzalez EA, Peng Z, Pati S, Park PW, Wang WW, Zaske AM, Menge T, Kozar RA. Modulation of Syndecan-1 shedding after hemorrhagic shock and resuscitation. PLoS ONE. 2011;6(8):e23530. doi: 10.1371/journal.pone.0023530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng Z, Pati S, Potter D, Brown R, Holcomb JB, Grill R, Wataha K, Park PW, Xue H, Kozar RA. Fresh frozen plasma lessens pulmonary endothelial inflammation and hyperpermeability after hemorrhagic shock and is associated with loss of syndecan-1. Shock. 2013;40(3):195–202. doi: 10.1097/SHK.0b013e31829f91fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hart ML, Ceonzo KA, Shaffer LA, Takahashi K, Rother RP, Reenstra WR, Buras JA, Stahl GL. Gastrointestinal ischemia reperfusion injury is lectin complement pathway dependent without involving C1q1. J Immunol. 2005;174:6373–6380. doi: 10.4049/jimmunol.174.10.6373. [DOI] [PubMed] [Google Scholar]
- 18.Ban K, Peng Z, Pati S, Witkov RB, Park PW, Kozar RA. Plasma-mediated gut protection after hemorrhagic shock is lessened in syndecan-1−/− mice. Shock. 2015;44(5):452–457. doi: 10.1097/SHK.0000000000000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper DK, Ekser B, Tector AJ. A brief history of clinical xenotransplantation. Int J Surg. 2015;23(Pt B):205–210. doi: 10.1016/j.ijsu.2015.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galili U. The α-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy. Immunology and Cell Biology. 2005;83:674–686. doi: 10.1111/j.1440-1711.2005.01366.x. [DOI] [PubMed] [Google Scholar]
- 21.Newman PJ, Aster R, Boylan B. Human platelets circulating in mice: applications for interrogating platelet function and survival, the efficacy of antiplatelet therapeutics, and the molecular basis of platelet immunological disorders. J Thromb Haemost. 2007;5(Suppl 1):305–309. doi: 10.1111/j.1538-7836.2007.02466.x. [DOI] [PubMed] [Google Scholar]
- 22.Pati S, Potter DR, Baimukanova G, Farrel DH, Holcomb JB, Schreiber MA. Modulating the endotheliopathy of trauma: Factor concentrate versus fresh frozen plasma. J Trauma Acute Care Surg. 2016;80(4):576–585. doi: 10.1097/TA.0000000000000961. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto M, Lin XH, Kominato Y, Hata Y, Noda R, Saitou N, Yamamoto F. Murine equivalent of the human histo-blood group ABO gene is a cis-AB gene and encodes a glycosyltransferase with both A and B transferase activity. J Biol Chem. 2001;276(17):13701–13708. doi: 10.1074/jbc.M010805200. [DOI] [PubMed] [Google Scholar]
- 24.Silliman CC, Ambruso DR, Boshkov LK. Transfusion-related acute lung injury. Blood. 2005;105(6):2267–2273. doi: 10.1182/blood-2004-07-2929. [DOI] [PubMed] [Google Scholar]
- 25.Pusateri AE, Given MB, Schreiber MA, Spinella PC, Pati S, Kozar RA, Khan A, Dacorta JA, Kupferer KR, Prat N, Pidcoke HF, Macdonald VW, Malloy WW, Sailliol A, Cap AP. Dried plasma: state of the science and recent developments. Transfusion. 2016 Apr;56(Suppl 2):S128–S139. doi: 10.1111/trf.13580. [DOI] [PubMed] [Google Scholar]


