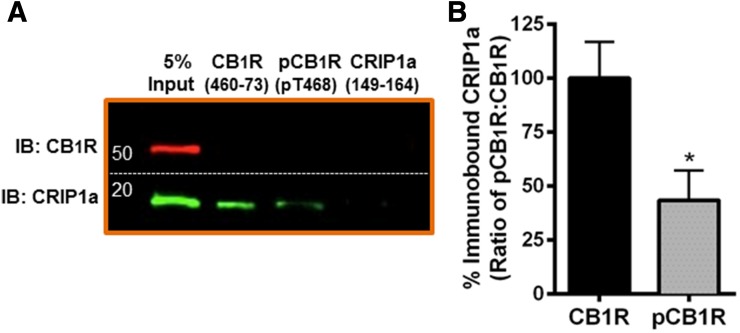
Fig. 4.
Pulldown of CRIP1a by a CB1R distal carboxy terminus peptide is attenuated by phosphorylation. (A) Pull-down of proteins using agarose beads coupled to peptides corresponding to the CB1R C-terminus, T468-phosphorylated CB1R C-terminus, or CRIP1a C-terminus. Bound proteins were washed, eluted, and subjected to immunoblot analysis as described in Materials and Methods. (B) CB1R C-terminus phosphorylation disrupts peptide association with CRIP1a. Quantification of CRIP1a immunoblot band densities is presented as binding to the nonphosphorylated CB1R expressed as 100%. Data are calculated from three independent experiments and are expressed as the mean ± S.E.M. *P < 0.001 indicates significant difference from non-phosphorylated peptide using Student’s t test.