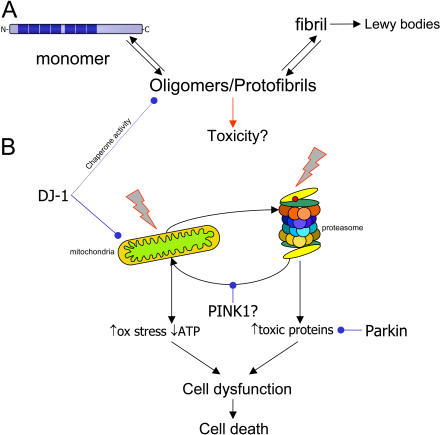
Figure 1. Molecules That Cause or Prevent Parkinson's Disease.
(A) shows a simplified, linear view of the aggregation pathway of α-synuclein (in blue). The monomer of α-synuclein is a natively unfolded protein with several repeats, shown by dark bars on the monomer. The protein has an innate tendency to aggregate with other molecules of α-synuclein, first into oligomers (also known as protofibrils), then into fibrils. It is the fibrillar forms of α-synuclein that are deposited into the classic pathological structures of PD, Lewy bodies. There are several studies that suggest that the oligomeric intermediates are the major toxic species, although this is not certain. (B) shows the recessive mutations associated with parkinsonism and their possible relationships to subcellular targets, either mitochondria (left) or the proteasome (right). Insults to either of these can cause cellular damage and may interact. For example, proteasome inhibitors can cause mitochondrial damage, which can be antagonized by PINK1. Parkin can promote the turnover of proteasomal substrates, and DJ-1 can prevent mitochondrial damage.
Quite whether (B) relates to (A) is not clear, but recent results with DJ-1 imply that DJ-1 has chaperone activity towards oligomers of α-synuclein (see text). Although there is much to be done to resolve the order of these events, it is likely that, either alone or in concert, damage to multiple cellular pathways leads to neuronal dysfunction and, eventually, cell death.