Abstract
Background:
Multimodality therapy constitutes the standard treatment of advanced and recurrent head and neck cancer. Since locoregional recurrence comprises a major obstacle in attaining cure, the role of intraoperative radiation therapy (IORT) as an add-on in improving survival and local control of the disease has been investigated. IORT allows delivery of a single tumoricidal dose of radiation to areas of potential residual microscopic disease while minimizing doses to normal tissues. Advantages of IORT include the conformal delivery of a large dose of radiation in an exposed and precisely defined tumor bed, minimizing the risk of a geographic miss creating the potential for subsequent dose reduction of external beam radiation therapy (EBRT). This strategy allows for shortening overall treatment time and dose escalation. The aim of this review is to summarize recent published work on the use of IORT as an adjuvant modality to treat common head and neck cancer in the primary or recurrent setting.
Methods:
We searched the Medline, Scopus, Ovid, Cochrane, Embase, and ISI Web of Science databases for articles published from 1980 up to March 2016.
Results:
Based on relevant publications it appears that including IORT in the multimodal treatment may contribute to improved local control. However, the benefit in overall survival is not so clear.
Conclusion:
IORT seems to be a safe, promising adjunct in the management of head and neck cancer and yet further well organized clinical trials are required to determine its role more precisely.
Keywords: head and neck cancer, intraoperative radiotherapy, review
1. Introduction
Head and neck cancer constitutes the eighth leading cause of cancer-related deaths worldwide. Its incidence varies widely among different geographical areas: in North America and the European Union, head and neck cancer accounts for 3% to 4% of all cancer diagnoses, while in developing countries its incidence is higher due to smoke and drinking habits in combination with poor socioeconomic status.[1]
Head and neck cancer encompasses diverse tumor types arising from different cell progenitors and anatomic sites with various anatomic barriers. Nevertheless, more than 90% are of the same histological type squamous cell carcinomas (SCCs), but even among these, further diversification exists with respect to risk factors, pathogenesis, and clinical behavior.[2]
The impact of head and neck cancer and its treatment on speech, swallowing function, and self-image can have a devastating psychologic and fiscal impact on afflicted persons as well as a considerable economic burden on the healthcare system. Despite recent advances in surgery and chemoradiotherapy, the overall 5-year survival remains in the region of 50% and is mainly influenced by disease stage, positive specimen margins, level of positive nodes, presence of extracapsular spread, perineural or lymphovascular invasion, encroachment of surrounding vital structures, and patient performance status.[3–5] Patterns of failure consist of recurrence at the primary tumor site or regional lymph nodes or a second primary or at distance sites such as the lungs, liver, and spine. Recurrence rates vary between 18% and 33% for advanced disease.[6]
The current treatment approach for locally advanced tumors of the head and neck is both surgery and radiotherapy (RT) or multimodality therapy incorporating chemotherapy and new agents such as epidermal growth factor receptor inhibitors. Two randomized studies that have been conducted by the European Organization for Research and Treatment of Cancer and the Radiation Therapy Oncology Group have demonstrated increased locoregional control when chemotherapy is added to radiation, but at the expense of increased toxicity.[4–5] In addition, there is evidence that when shortening treatment duration from the end of the surgery to the end of radiation in high-risk patients, there is a significant increase in locoregional control and survival (3–13). Given that approximately only 15% to 30% of patients present with early-stage disease, whereas 60% to 80% present with locoregionally advanced disease, one of the main challenges for treatment of head and neck cancer is to prevent or curb locoregional recurrence of advanced disease since there are indications that long-term control of local disease may influence patient survival.[7–12]
Recurrences after irradiation may be addressed by salvage surgery if resection is possible, plus additional chemoradiation. Nevertheless, severe complications may occur due to limited tolerance of the previously irradiated tissues to reirradiation. In reported series of patients with head and neck cancer who underwent full-dose reirradiation, 21% developed mucosal necrosis, 8% developed osteoradionecrosis, and 3% experienced fatal carotid artery blowout.[14] Recently, new RT techniques such as intensity modulated RT (IMRT) and stereotactic body RT (SBRT) have improved oncological results with reduced toxicities but specific indications have not been defined yet.[15–16] Reirradiation still poses a major challenge for the radiation oncologist.
In this context, IORT is an alternative to be considered, not only to achieve local control of advanced or residual disease, but also as adjuvant therapy in salvage surgery. IORT was pioneered in the 1960s by the Japanese for treatment of gastrointestinal tumors and introduced in the United States and Europe in the 1970s, initially for abdominal and gynecologic malignancies.[17–18] IORT can be used as a boost to external beam radiation or as the sole irradiation modality in a previously irradiated field. IORT allows the early and rapid delivery of large single doses of radiation to a visible tumor bed with exclusion or shielding of critical normal structures from the treatment field.[19] IORT is generally delivered from a linear accelerator using mainly an electron beam field or in some cases a photon beam field. The field is well visualized, which allows for relatively easy placement of the electron beam or the photon beam cone on the tumor bed. This allows a steep dose fall off while sparing normal tissues.[20] Occasionally, IORT is combined with external radiation therapy (EBRT) to provide the best combination of local and locoregional treatment.
A theoretical advantage of IORT is the decreased possibility of geographical miss when radiation is delivered at the time of surgery and the increased probability of the “sterilization” of stem cell since radiation is delivered at a minimum cell number. There is also increased biological efficacy per unit dose because of the administration of radiation as a single fraction with no time elapsing between multiple fractions and no time elapsing between surgical excision and RT. In addition, IORT can decrease the overall treatment time by reducing tumor cell repopulation during treatment. IORT allows dose escalation because it is estimated that the single high dose delivered by IORT is biologically equivalent to 2- to 3-fold that of conventional EBRT, allowing a reduced dose of EBRT to treat microscopic disease at lower risk areas. IORT toxicity does not overlap with that of EBRT. When properly combined with EBRT, IORT can be used for dose escalation while potentially decreasing toxicity.[21–25]
In this article, we review the efficacy, effectiveness, and safety of IORT for the treatment of advanced or recurrent head and neck cancer and discuss its potential applications.
2. Methods
The information for this Review was compiled by using Medline, Scopus, Ovid, Cochrane, Embase, and ISI Web of Science databases for articles published from 1980 through March 2016. Electronic early-release publications were also included. The search terms used included IORT, IOERT, intraoperative RT, intraoperative radiation, head and neck cancer. Articles published in any language other than English, Spanish, Italian, or French were excluded. When possible, primary sources have been quoted. References were chosen on the basis of the best clinical or technical evidence and selected on the basis of the following inclusion criteria: in terms of study design, selection was restricted to systematic reviews, meta-analyses, clinical trials, cohort studies, case–control studies, and case series; in terms of sample size, there was no restriction in number of patients who received treatment with IORT; in terms of the target disease, adult patients with head and neck cancer of any histology and extension were eligible; finally, in terms of survival, we included studies that assessed survival with a mean or median follow-up of 3 months or longer. Since assessed papers were mainly case series with paucity of randomized controlled trials, stated conclusions should be judiciously interpreted until randomized studies are published.
Notice: an ethics committee/institutional review board approval or patient consent was not applicable for this study because it is a systematic review work.
3. Results
Twenty-one case series studies fulfilled our selection criteria. All studies were allocated and analyzed by the years of trial rather than the publication date, beginning with the oldest one.
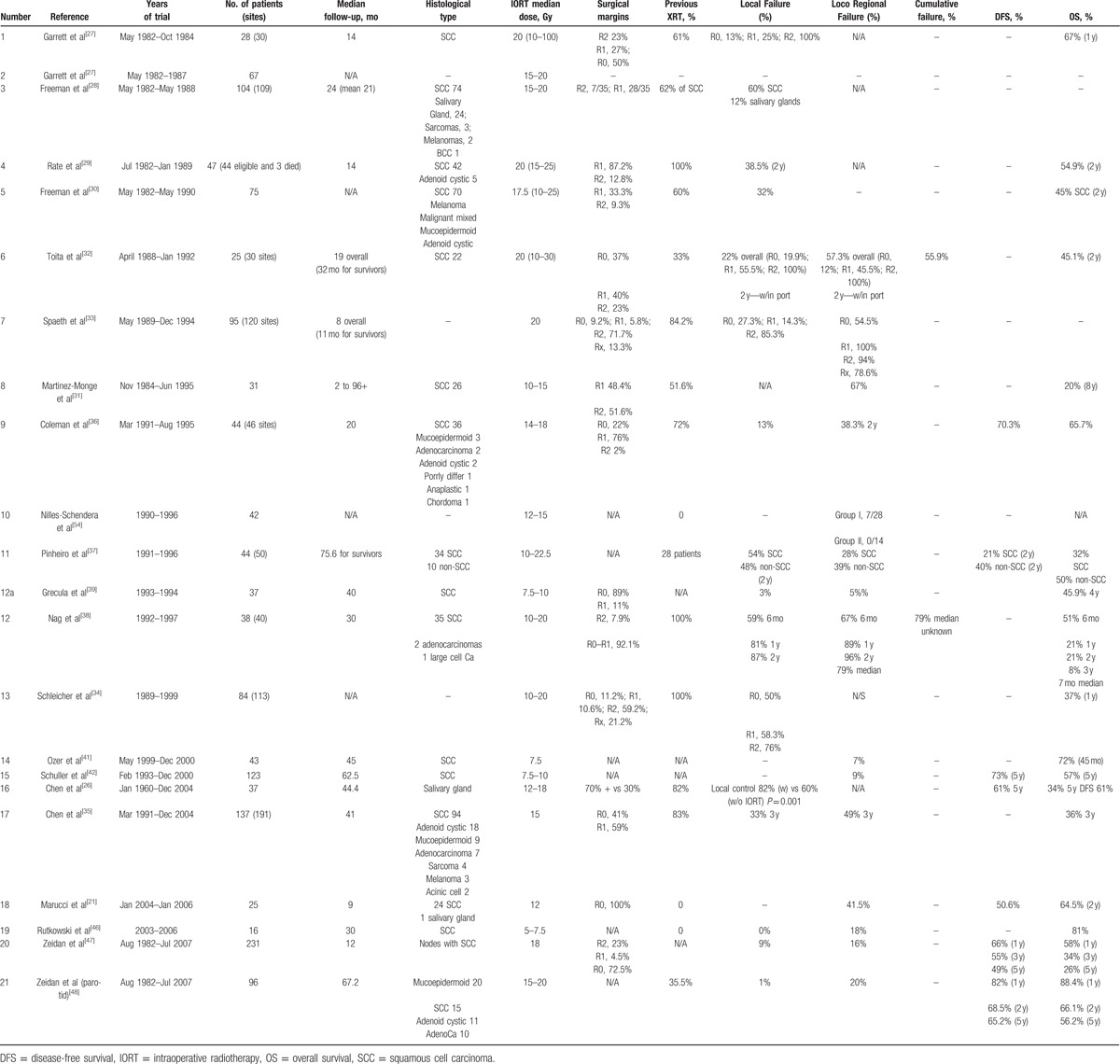
All patients studied received IORT for either locoregionally advanced disease or recurrent disease. Basic quality features of the aforementioned studies are tabulated in Table 1. Moreover, histologic type, the number of patients, median dose of IORT used, percentage of positive versus negative specimen margins, locoregional failure rates, and overall survival (OS) are recorded.
Table 1.
Main clinical features of the published studies.
Chen et al[26] studied a cohort of 99 patients with locally recurrent salivary gland malignancies from January 1960 to December 2004. It is noteworthy that this study represents the longest running one in terms of data collection and mean follow-up periods that spanned 40 years and 44.4 months, respectively. However, IORT was first utilized in 1991, with 37 out of 99 patients being candidates; 5 patients of this subgroup received additional EBRT. Median dose was 15 Gray (Gy) (12–18 Gy). A total of 32 patients developed a subsequent recurrence, 15 of which were isolated events without disease progression elsewhere. The median time to second recurrence was 1.3 years. The local control was 82% when IORT was used, whereas a rate of 60% was succeeded when IORT was abandoned (P = 0.001), albeit 69 patients (70% of total) had positive margins. The 1-, 3-, and 5-year estimates of local control were 88%, 75%, and 69%, respectively. OS for the entire patient population at 1, 3, and 5 years were 83%, 54%, and 34%, respectively. Patients with locally recurrent adenoid cystic carcinoma had a 5-year OS of 67%. Disease-free survival (DFS) at 1, 3, and 5 years were 69%, 57%, and 46%, respectively. The 5-year DFS estimates for patients treated with IORT were 61% and without IORT they were 44%. Statistical analysis revealed that IORT was an independent predictor for local control and DFS, but not for OS, since histology and the high rate of distant metastases (42%) governed life expectancy, with the exception of adenoid cystic carcinoma which showed a less-ominous behavior. No perioperative serious complications were reported. The main toxicities that were presented are superficial wound infections (2 patients), trismus (1 case) that resolved 1 year later, and facial pain secondary to neuropathy (1 case) which was managed medically. The authors based on the results of their study concluded that after salvage surgery for locally recurrent salivary gland carcinomas, the addition of IORT results in significantly improved local control and DFS compared with resection alone.
Garret et al[27] treated 28 patients (30 treatment sites) suffering from advanced head and neck SCC with a minimum follow-up period of 14 months. There were 3 indications for the use of IORT. Gross residual disease in 7 sites, microscopic residual disease in 8 sites, and close resection margins in 15 sites. A total of 61% of the patients had received previous external beam irradiation with a median dosage of 60 Gy. IORT median dose was 20 Gy. Local failure was noticed in 13% (2 out of 15) of the cases with close surgical margins, while there was a 25% local failure rate in patients treated for microscopic residual disease (2 out of 8). All patients with gross residual disease developed recurrence in the surgical field. Thus, local control was significantly higher (78%) in cases with close and microscopic residual margins versus residual disease (P < 0.02). In addition, there was a lower rate of local failure in cases that had been previously irradiated: 35% versus 40% in nonprior irradiated patients. The actuarial l-year survival for all patients treated with IORT was 67%. For close surgical margins, the l-year survival was 76%, and for microscopic disease it was 86%. The actuarial l-year survival for patients with gross residual disease was 14%. None of the patients with gross residual disease survived. The main serious complications that were noticed were carotid blowout (2 cases) and osteonecrosis of the mandible (1 case). The authors suggested that in order for IORT to be successful, all gross disease must be resected. They also reported that coverage of the carotid region with a myocutaneous flap may prevent major complications.
Freeman et al[28] reported a series of 104 patients treated with surgery plus IORT for miscellaneous aggressive primary or recurrent head and neck tumors. Seventy-four tumors were epidermoid SCC of the upper aerodigestive tract, 24 were salivary gland tumors, while there were 3 cases of sarcomas, 2 melanomas, and a single case of extensive recurrent basal cell carcinoma. A total of 62% of the patients suffering from SCC had received previous external beam irradiation. The locoregional control was estimated based on tumor site, histology, and surgical margin. The dosage was usually 20 Gy to areas in the neck and 15 Gy to areas in the oral cavity, salivary gland, and skull base. The site-adjusted recurrence control regardless of histologic type was 47% in the neck, 50% in the skull base, 69% in the parotid, 57% in the tongue, 33% in the temporal bone, 100% in the mandible, and 33% in the floor of the mouth. Tumor histology and margins were also strong predictors of local control. Moreover, a 2-year follow-up in 35 out of 74 patients with SCC showed that a 40% local control was achieved, and patients with microscopic disease or close margin fared better. In addition, a 100% local control was recorded for adenoid cystic, adenocarcinoma, and moderate-grade mucoepidermoid after a median follow-up of 23, 26, and 24 months, respectively. More aggressive salivary tumors, such as malignant mixed tumor and high-grade mucoepidermoid were more refractory to treatment with local control of 67% and 71% at 23 and 12 months, respectively. The most serious toxicity were the following: osteonecrosis (n = 3), fistulas (n = 6), rupture of the carotid, or innominate arteries (n = 3). Authors opined that IORT was a promising treatment modality, but OS and DFS were not reported.
Rate et al[29] studied 47 patients suffering from recurrent SCC (42 patients) and adenoid cystic carcinoma for the interim between 1982 and 1989. Initial treatment modalities were XRT alone for half of these patients and surgery plus EBRT for the remainder. All patients underwent surgery plus IORT as salvage treatment. The dose delivered was 15 Gy (18 patients), 20 Gy (28 patients), or 25 Gy (1 patient) based on the proximity of index tumor to vital structures. The median follow-up was 14 months. The total 2-year actuarial survival was 54.9%, but the 2-year survival for patients with SCC was 57.2%. Of note, there were no significant differences in survival and local control between patients with primary site recurrence and those with node failure. Again, gross residual disease portended a dismal prognosis when compared to microscopic disease (local control 20% vs 57.9%, P = 0.05) (OS 16.7% vs 46.3%, P < 0.09). They concluded that a combination of surgical resection and IORT was an effective treatment modality for recurrent head and neck cancers in previously irradiated fields. Nevertheless, the limitation of this analysis was the small number of patients treated with gross residual disease. In addition, serious complications emerged: pulmonary insufficiency (n = 3), osteoradionecrosis (n = 2), fistula formation (n = 2), and 1 patient with gross tumor infiltration of the carotid artery died of a carotid blowout.
Freeman et al[30] reported the results of a series of 75 patients treated with a combination of IORT and surgical resection between May 1982 and December 1990. Indications were advanced neck metastasis in all patients, while 70 of them had SCC and the remaining had other tumors. Treatment was for disease recurrence in 52 patients (46 of them had previous irradiation) and for the rest IORT was incorporated in their initial treatment. A total of 25 of the 75 patients received postoperative irradiation. Surgical margins examination revealed microscopic disease in 25 patients (33.3%), while gross macroscopic disease was present in 7 patients (9.3%) after resection. IORT dosage ranged from 10 to 25 Gy. The overall IORT control rate was calculated to be 68% in 38 patients who did not die of either recurrent disease that was exclusively outside the IORT port or other concurrent disease during the first 2 years of follow-up. IORT did not seem to benefit patients with gross evidence of residual cancer in the margins; only 1 (25%) of those patients maintained control in the IORT port for at least 2 years. The OS rate was 45% in 2 years for patients with SCC. Survival rate was strongly related to surgical margins status. Patients with gross residual disease had a 2-year survival rate of 14% compared to those with microscopic free margins who had a 2-year survival rate of 44%. Major complications occurred in 19 (25%) of the patients. There were major vascular ruptures (n = 5), pharyngocutaneous fistulas (n = 4), and neurological problems (n = 6). Other complications included sepsis, flap necrosis, pulmonary embolus, myocardial infarction, and hypocalcemia. Researchers concluded that survival and local control rates might be improved in patients with advanced cervical metastasis by incorporating IORT with aggressive resection. However, IORT was not effective in controlling residual disease that was evident on gross examination.
Martinez-Monge et al[31] presented their results of a series of 31 patients with locally advanced or recurrent disease treated with surgery plus IORT and pre- or postoperative EBRT in the period from November 1984 to June 1995. Predominant histology was SCC in 83.8%. Preoperative chemotherapy was added in 5 patients, while 16 patients had been previously irradiated for primary disease. IORT treatment was used to the primary site in 42.5% of the cases and to regional nodes in 57.7%. Dose per target volume was in the range of 10 to 15 Gy. Gross residual disease remained in 51.6%, while microscopic residual disease was found in 48.4%. Local and/or regional failure occurred in 2/3 of patients. The 8-year survival was 20% (range 2 to 96+ months), while the median survival time was only 14 months. However, 3-year local control was 50% in a subset of 5 patients with primary N3 disease treated with the addition of induction chemotherapy. Distant metastases were noticed only in 18.5% of the patients. Macroscopic residual disease and inability to deliver full-dose adjuvant EBRT were considered to be the most significant factors of locoregional failure and OS. Patients who were unable to receive full-dose adjuvant external RT because of prior radiation therapy had a median survival time of 6 months, (P = 0.04) and patients with presence of macroscopic residual disease had median survival time of 8 months (P = 0.029). Reported complications were pharyngeal fistula (n = 5), graft placement failure (n = 1), and nerve injury (n = 1). The investigators concluded that IORT as an adjuvant to head and neck radical surgery adds little toxicity if used in the dose range 10 to 15 Gy but is not effective in cases of gross residual disease that were treated by prior external beam irradiation and cannot be fully reirradiated.
Three years earlier, Toita et al[32] presented a study of 25 patients with 30 involved sites. The total study duration spanned almost 4 years, from April 1988 to January 1992. The vast majority (22/30) of the cases implicated locoregional failure that had been previously treated by surgery and/or XRT. In 10 of those 22, EBRT had been used. Among those 30 sites, surgery plus IORT (10–30 Gy) were given to 9 primaries and 21 metastatic nodes mainly fixed to the carotid artery. Evaluation of surgical specimens revealed gross residual disease in 7 sites, microscopic residual disease in 12 sites and close margin in the remainder. Pre- or postoperative EBRT (10–70 Gy) was given in 20 out of 30 sites. The median follow-up period for all patients was 19 months and 32 months for surviving patients, with 2 patients being lost to follow-up. Again SCC was the prevalent histology involving 22 out of 25 patients. The 2-year local control rate was 0% for R2, 54.5% for R1, 81.1% for close margins, and the cumulative was 54.1%. There were significant differences between gross residual disease and microscopic disease (P < 0.05) and gross residual disease and close margin (P < 0.01). Likewise, the control rate for primary sites was superior to that of metastatic nodes. However, this difference was insignificant. A total of 5 patients developed lung metastasis after 2 to 17 months. Four of them had gross disease. The 2-year cumulative survival rate was 45.1% for all, 0% for R2, 33% for R1, and 70% for R0. Again, there were significant differences between gross residual disease and microscopic disease (P < 0.05), as well as gross residual disease versus close margins disease (P < 0.01). Authors also noticed that infield local failure was accompanied by regional failures outside the port. The latter were ascribed to inadequate coverage and doses or absence of external irradiation combined with IORT. Five patients developed lung metastasis after 2 to 17 months. Four of them had gross disease. The most serious complications that were developed were osteoradionecrosis (n = 4) and carotid artery blowout (n = 3). Two of these were fatal. The incidence of complications increased when a single dose of IORT over 20 Gy was delivered. Thus, the authors discussed the necessity of considering not only local control, but also late complications for determining the optimum dose of IORT. The authors stated that IORT is not effective in the treatment of locally advanced or recurrent head and neck cancer. In cases of microscopic disease or close margins disease, they could not state whether IORT added therapeutic value in controlling locoregional disease compared with conventional postoperative irradiation alone.
Concurrently, Spaeth et al[33] evaluated the palliative efficacy of IORT treatment from May 1989 to December 1994, in a total of 120 sites in 95 patients predominantly suffering from recurrence in lymph nodes (75.8%) and primary site (11.7%). To a lesser extent, IORT constituted part of the initial treatment (12.5%). IORT patients had to meet 1 or more of the following criteria: previously performed full-course radiation of the area of malignant tissue (usually within the last 3–5 years); unresectable fixation of the tumor to vital structures, R0 or at least R1 resection; ulceration of the tumor; pain; and other contraindications against radical surgical treatment. Most of the tumors were classified as SCC, with larynx, oro-, and hypopharynx being the most common primaries. Prior treatment included surgery alone (6.3%) or in combination with EBRT and chemotherapy (77.5%). Thirteen (16.3%) patients had only received EBRT or combined chemotherapy and EBRT. Together, these factors alluded to the aggressiveness of the tumor. Of note, 70% of the patients had already reached stage IV, and as a high-risk group had little chance of complete remission. A total of 84.2% of the patients received a single dose of 20 Gy (range10–40 Gy). Sites treated with IORT were classified into R2 = 86 = 71.7%, R1 = 7 = 5.8%, R0 = 11 = 9.2%, and 16 patients (13.3%) could not be definitively classified into one of those groups. Nevertheless, local control rate was attainable in 16.7% (R2 resection), 85.7% (R1 resection), and 72.7% (R0 resection), with a mean 11-month follow-up period for survivors, but only 8 months for deceased patients. In 87% of patients, pain was reduced or at least a further increase could be avoided for weeks to months, as these patients’ quality of life could be improved at least for a limited period of time. No major complications were reported, and the toxicity was limited to wound healing disorder (n = 8), fistulas (n = 3), and partly due to comorbidities such as diabetes mellitus. The authors suggested that IORT was beneficial to the patients in improving pain control and quality of life but the increase of OS, although occasionally attainable, was not a major goal.
Four years later, Schleicher et al[34] reiterated the same protocol (IORT palliative effect) in a cohort of 84 patients exclusively treated for recurrent head neck cancer for a total of 113 sites. Hence, alleviation of symptoms such as tumorous swelling, pain, ulceration, dysphagia, dyspnea, bleeding, and fistula was the primary treatment goal. All patients had undergone at least 1 course of EBRT. IORT was delivered at the jugular lymphatic chain in 80 cases, followed by the supraclavicular pit in 8 cases, the larynx in 7 cases, and 18 treatments located in other different head and neck regions. The median recurrence interval was 38.3 weeks (range: days–9.7 years). Mean IORT dosage was 20 Gy. Surgery yielded poor disease control as R2 margins were found in 59% of the patients, and most of them (51 out of 67) experienced local recurrence. Failure rates were about 50% in R0 and R1 resections, respectively. The mean OS time was 11.4 months with a maximum time of 7 years. Patients after R0 resection showed a significantly (P = 0.03) longer survival (median, 15 months) than those after R1 or R2 resection (6.3 months). Lack of aggressive salvage surgery warranted the particularly low survival rates (1 year, 37%). However, pain alleviation was noted in 70.7% of the patients and was independent of resection status. The complication rate was comparable to the one expected after surgery alone, and no major complications were reported. They were wound healing complications (n = 10), infections (n = 5), salivary fistulas (n = 4), and skin necrosis (n = 2). The authors reported that all of the patients experienced relief from their symptoms, and they conclude that IORT can be well integrated into a palliative treatment concept for large tumors with poor prognosis irrespective of resection status.
According to Chen et al,[35] in a series of 137 patients treated with IORT for recurrent head and neck disease from March 1991 to December 2004, 3-year rates of locoregional control, distant metastasis-free survival, and OS were 51%, 46%, and 36%, respectively. Initially, surgery was the main treatment modality in 108 (79%) patients and EBRT in 29 (21%). However, a total of 113 (83%) patients had previously undergone a full course of EBRT. SCC was the predominant pathology occurring in 94 patients (69%), followed by salivary gland tumors in 36 patients (26%) and other more rare tumors. The oropharynx (24%) and the oral cavity (23%) were the most frequently affected locales followed by paranasal sinus (12%), parotid gland (12%), hypopharynx (7%), submandibular gland (6%), skin (6%), nasopharynx/nasal cavity (3%), larynx (3%), ear (2%), and 2% unknown. Regional failures alone or in conjunction with regression at primary site were found in 39 and 11 patients, respectively. In 87 patients, an isolated local failure was noted. Median relapse time was 13 months (range 4–107 months). Retreatment plan included salvage surgery plus IORT to a median dose of 15 Gy. Resection margins status was R1 in 56 patients (41%) and R0 in 81 patients (59%). Adjuvant chemotherapy was administered in 99 patients, and 35 of those patients, 11 of whom had been previously irradiated, received additional post-IORT EBRT. The median follow-up was 41 months (among surviving patients). Overall survival at 1, 2, and 3 years were 68%, 52%, and 36%, respectively, and multivariate analysis revealed that only the involved site (primary vs neck) was predictive, albeit the benefit of adjuvant chemotherapy was not estimated. The 1-, 2-, and 3-year estimates of locoregional control for the entire patient population were 68%, 61%, and 51%, respectively. In addition, patients who received IORT at the primary site had significantly better survival than those who were treated with IORT for disease involving the neck. Between these 2 sites, 3-year OS were 44% and 19% (P = 0.001), and the median survival was 20 and 12 months, respectively. Moreover, 15/87 of the patients treated with IORT for local disease developed regional metastases at out-of-field sites after a median 10-month interim; 9 were solitary, and the other 6 occurred along with distant metastasis. When neck was treated, 15 out of 50 patients relapsed (6 isolated, 2 locoregional, and 7 regional + distant) at a median of 3 months. The 1-, 2-, and 3-year estimates of in-field control were 76%, 69%, and 67%, respectively. The only parameter predictive of in-field recurrence was positive microscopic margins at the time of salvage surgery and IORT. Hence, the 1-, 2-, and 3-year rates of IORT in-field were 87%, 82%, and 82% for patients treated with negative surgical margins, while for patients with positive margins they were 65%, 53%, and 48%, respectively (P = 0.002). IORT also offered a significant benefit in obviating distant metastasis as patients treated with IORT to the primary tumor site had a 3-year distant metastasis-free survival of 61% compared with the survival rate of 30% for those who were treated with IORT to disease sites in the neck (P = 0.001). Complication rate was relatively low as only 9 out of 137 patients were affected and they were wound infections (n = 4), orocutaneous fistulas (n = 2), flap necrosis (n = 1), trismus (n = 1), and neuropathy (n = 1). The authors conclude that IORT may enhance salvage surgery results in controlling recurrent or persisting head and neck cancer in appropriately selected patients.
Coleman et al[36] reported their 4 1/2-year experiences of IORT after studying a cohort of 44 patients (46 sites) for recurrent or locally advanced head and neck cancer from March 1991 through August 1995. The indication for IORT in 78% of the cases was persistence of primary tumor after definitive therapy or 1 or more recurrences, and in 22% the indication was extensive primary disease with risk factors for local failure. The median follow-up was 20 months. The main pathology was SCC in 36 sites (78%) followed by salivary glands tumors in 7 sites (15%) and other rare tumors in the rest of the patients. Thirty-three patients (72%) had previously received external beam irradiation. The doses of IORT ranged from 14 to18 Gy. Four patients received chemotherapy postoperatively. The IORT-related surgery revealed R2 = 1 = 2%, R1 = 35 = 76%, and R0 = 10 = 22%. There were 19 relapses, 14 of which were locoregional. Six local recurrences (13%) occurred, 3 associated with regional failure as well. Eight were regional recurrences in the surgical bed but outside the EBRT-IORT field and 1 was a regional failure accompanied by distant metastases. Four failures were distant metastases only. Sixteen patients deceased, but only 2 with in-field recurrence. The actuarial 2-year locoregional control rate was 61.7%. The actuarial 2-year survival was 65.7%, but for disease-related mortality the 2-year survival was 70.3%. Complications came about in 24% of the cases. These included mucositis (n = 1), supraglottic edema (n = 1), temporomandibular joint abscess (n = 1), osteoradionecrosis (n = 1), and wound dehiscence (n = 1). The most serious complications that were noticed were carotid rupture (n = 1), facial nerve palsies (n = 3), stroke (n = 1), and carotid syndrome (n = 1). The authors suggested that IORT, when combined with postoperative external beam irradiation and salvage surgery, appears to improve the local control of high-risk primary or recurrent tumors. Yet, there is no benefit regarding regional and distant failures. Nevertheless, complication rates of IORT are low, adding little to patient morbidity.
In Pinheiro et al[37] study, the impact of IORT as an adjuvant treatment of advanced head and neck skull base cancer was evaluated. There were 44 candidates with the involvement of 50 sites treated between 1991 and 1996. A total of 34 patients with SCC and 10 patients with non-SCC were registered. Previous treatment included surgery, RT, and chemotherapy, but there was no exact reference of particular treatment applied to each patient. Skull base (56%) and neck (44%) comprised the sites treated with IORT. Actual doses delivered were 10 to 20 Gy, and 1 field was treated to 22.5 Gy. Paradoxically, patients with SCC had similar central and local failure rates at 2 years (54%) as patients with non-SCC (48%, P = 0.67) and distant failure (51% vs 35%, P = 0.74) and regional recurrences (28% vs 39%, P = 0.52), with almost similar trends being recorded at 5 years. Again, gross disease was associated with higher locoregional failure rates, with R1 and R0 sharing statistically similar rates (62% vs 43%, P = 0.24). However, among the latter, distant metastasis was more commonly seen in patients with R1 margins than those with R0 margins (2-year rate of distant failure of 54% vs 39%, P = 0.1). Despite the aforementioned parities seen between these 2 groups, non-SCC patients fared better at 2 years as OS and DFS were 50% and 40%, respectively, whereas values of the same parameters in SCC-patients were 32% and 21%, respectively. Authors beheld that this difference in survival possibly reflects the different natural history of these 2 diseases rather than a greater radiosensitivity of non-SCC. The toxicity developed was soft tissue minor complications (n = 5), fistulas (n = 3), neurologic complications (n = 5), dysphagia (n = 2), trismus (n = 2), skin and wound complications (n = 4), bone pain (n = 1), Eustachian tube dysfunction (n = 1), and fatal carotid hemorrhage (n = 1). The authors conclude that IORT used in doses less than 20 Gy is safe in treating advanced head and neck and skull base cancer even in previously irradiated patients. It may be useful in cases of advanced cancer, especially at the skull base, where microscopic residual tumor is likely even after a “complete” surgical resection.
Between January 1992 and March 1997, Nag et al[38] retrospectively evaluated the efficacy of palliative surgery plus IORT in 38 previously irradiated patients. Seven patients (18%) had prior chemotherapy and 29 patients (76%) had previously undergone 1 or more surgical procedures. SCC had been diagnosed in 92% of the cases. Larynx and oral cavity tumors had been the most common primaries. IORT was delivered in 18 patients with first recurrence, 13 with a second one, and 7 during subsequent relapses. R2 margin involved 3 patients, and R1/R0 margins remained in 35 patients. Hence, 34 patients with R0 or R1 received 15 Gy, 1 patient with R2 received 20 Gy, 2 patients with R0 received 10 Gy because of high (>70 Gy) EBRT doses, and 1 patient received 25 Gy to the area of gross residual and 15 Gy to the surrounding areas of microscopic residual. Median follow-up was 30 months, and 66% of patients developed recurrences within field during this period. The 6-month, 1-, and 2-year control rates were 41%, 19%, and 13%, respectively, with median of 6 months. Also, the 6-month, 1-, and 2-year locoregional control rates were 33%, 11%, and 4%, respectively, with a median of 4 months. The 6-month, 1-, 2-, and 3-year actuarial survival rates were 51%, 21%, 21%, and 8%, respectively. Interestingly, patients treated to neck sites had a better OS (4 of 14 alive) than those with primary (2 of 11), stomal (1 of 8), or multiple recurrences (0 of 6), P = 0.0054. They deduced that IORT alone does not provide good control of recurrent previously irradiated head and neck cancers. Finally, 16% of the patients had complications and included fistulae (n = 2), tracheal dehiscence (n = 1), wound dehiscence (n = 1), carotid occlusion (n = 1), and fatal tracheovascular fistula (n = 1).
A Multimodal Intensification Therapy including IORT for Previously Untreated Advanced Resectable Squamous Cell Carcinoma of the Oral Cavity, Oropharynx or Hypopharynx has been evaluated in the Ohio State University. Several pilot studies have been conducted with modifications of the scheme in each one in order to reduce systemic toxicity. From February 1993 through July 1994, Grecula et al[39] studied a series of 37 patients with advanced resectable head and neck SCC, involving mainly oral cavity, oro-, and hypopharynx. The median follow-up was 40 months. Eligibility criteria included the following: untreated resectable, clinical stage III, or IV disease (or stage II hypopharyngeal carcinomas), Karnofsky performance index of ≥60, adequate bone marrow function, serum creatinine ≤1.3 or creatinine clearance >60 mL/min, and normal liver function. Therapeutic scheme included neoadjuvant chemotherapy and preoperative EBRT followed by surgical resection, IORT, and postoperative EBRT along with 2 cycles of adjuvant chemotherapy. Overall compliance was 73%. No evidence of residual microscopic tumor at the surgical margins was achieved in 89% of the permanent section. Subsequently, patients with negative margins received a modest IORT dose of 7.5 Gy, while a 10-Gy dose was given when positive margin was left. The overall local control, regional nodal control, distant metastasis-free rates were 97%, 95%, and 81%, respectively. However, in fully complied patients, the corresponding rates were 100%, 96%, and 81%, respectively. The 4-year OS rate was 45.9%. This protocol achieved the most prominent results in terms of survival and locoregional control, but problematic compliance and high complication rates both acute and late, probably due to chemotherapy, were noted.
Recently, Most et al[40] evaluated the feasibility of flap reconstruction, after submitting 21 patients (receiving 22 treatments) to IORT for advanced and recurrent head and neck cancer. Sixteen patients had SCC of the upper aerodigestive tract or cervical nodes, 2 had poorly differentiated parotid adenocarcinoma, 1 had high-grade parotid mucoepidermoid carcinoma, 1 patient had a recurrent synovial cell sarcoma of the parapharyngeal space, and 1 patient had an advanced cutaneous SCC of the nape of the neck. All but 1 patient received prior treatment, included surgery in all cases, XRT for 21, and chemotherapy to 9 patients. Postoperative EBRT was given in 6 patients. The IORT dose ranged from 10 to 15 Gy, with median dose 12.5 Gy. Five of 22 cases (22.7%) had positive margins at the time of IORT. Report of OS and local relapse-free survival were omitted. Five patients had medical complications including Clostridium difficile colitis and stress gastritis (n = 1), transient myocardial ischemia (n = 1), deep vein thrombosis (n = 1), sepsis (n = 1), myocardial ischemia (n = 1), and postoperative pneumonia (n = 1). Authors concluded that IORT did not hamper flap viability.
Ozer et al[41] reported the results of a multimodal intensification regimen, applied from May 1999 to Dec 2000, in 43 previously untreated patients with resectable SCCs of the oral cavity, oropharynx, or hypopharynx. Median follow-up time was 45 months. Eligibility criteria included the following: Karnofsky performance index of ≥60, adequate bone marrow function, serum creatinine ≤1.3 or creatinine clearance >60 mL/min, and normal liver function. Fifteen patients had oral cavity primary cancers, 20 oropharyngeal, and 8 hypopharyngeal tumors. A total of 28% of patients (12 of 43) had stage III clinical disease at presentation, while 72% (31 of 43) had stage IV disease (without distant metastases). Therapeutic scheme included neoadjuvant chemotherapy and preoperative EBRT followed by surgical resection, IORT, and postoperative EBRT along with 2 cycles of adjuvant chemotherapy. Patients with negative surgical margins received an IORT dose of 7.5 Gy. Total protocol compliance was 53% (23 of 43 patients). Overall locoregional control was 93%, while the rate of distant metastases was 9%. Survival rates were high, with 72% of the patients being alive without evidence of disease and 7% alive with evidence of cancer. A total of 7% of the patients died of disease in the follow-up period. Acute and late toxicity rates were improved compared to previously reports from the same group due to chemotherapy scheme modifications. Operative complications rate did not differ from the usually reported for operations without perioperative chemoRT. The authors concluded that this multimodal intensification regimen not only demonstrated an improvement in patient and protocol compliance, but also achieved an excellent locoregional and distant metastatic disease control. However, since it was a pilot trial, further trials are necessary to validate the efficacy of this regimen.
Schuller et al[42] published the 12-year experience of the multimodal intensification regimens used in Ohio University for advanced, resectable, previously untreated SCC of the oral cavity, oropharynx, or hypopharynx. This study reported the overall toxic effects, compliance, long-term systemic and local disease control rates, and survival analysis associated with all intensification regimens completed in this center.[43–45] A total of 123 patients were registered in 3 consecutive intensification trials between February 1993 and December 2000. Median follow-up time was 62.5 months. Eligible patients had previously untreated, resectable SCC of the oral cavity, oropharynx, or hypopharynx (stage III or IV disease of the oral cavity and oropharynx and stage II, III, or IV disease of the hypopharynx), with no distant metastases. A total of 37 patients (30.0%) had oral cavity primary cancers, 54 (43.9%) had oropharyngeal cancers, and 32 (26.0%) had hypopharyngeal tumors. Most patients (77.2%) had stage IV disease. A Karnofsky performance index of 60 and greater, adequate bone marrow function (platelet count 100–109/L and absolute neutrophil count 2.0–109/L), creatinine clearance greater than 1.0 mL/s (60 mL/min), adequate hepatic function (bilirubin level 1.8 mg/dL [31 μmol/L]), and serum transaminase levels less than 4 times the upper limit were required. Therapeutic scheme included neoadjuvant chemotherapy and preoperative XRT followed by surgical resection, IORT, and postoperative EBRT along with 2 cycles of adjuvant chemotherapy. Patients with negative surgical margins received an IORT dose of 7.5 Gy, while patients with positive margins received 10 Gy. Total protocol compliance was 60.9% (75 of 123 patients). The overall locoregional disease control rate was 91% (112/123). The rate of distant metastases was 13.8% (17/123). Although overall 5-year survival was 57%, disease-specific 5-year survival was 73%. Operative complications rate was consistent with surgical complication frequencies in the absence of perioperative chemoRT. The authors stated that part of the success in disease control and survival in the intensification regimens was attributed to IORT.
In Marucci et al[21] study, 25 patients were enrolled and deemed after receiving IORT as an “early boost” for advanced locoregional disease, from January 2004 to 2006. Included patients had resectable locally advanced head and neck cancer, primary or recurrent neoplasms without previous irradiation. Twenty-four had SCC, 1 had salivary carcinoma, 17 patients had stage IV disease, and the remainder had tumor relapse. Oral and skin cancers represented the most common diagnoses. The median follow-up time was 9 months. IORT dose of 12 Gy was delivered to all patients after complete tumor resection, (R0 = 100%), while 20 of them also received postoperative adjuvant EBRT. The 2-year OS was 64.5%, locoregional relapse-free survival was 58.5%, and DFS was 50.6%. There were 6 cases of death due to deteriorating general condition (n = 2), respiratory infection (n = 1), systemic progression (n = 1), locoregional recurrence (n = 1), and carotid blowout (n = 1). The minor complications were fistula (n = 2), fistula with partial flap necrosis (n = 1), hematoma (n = 1), partial flap necrosis with wound dehiscence (n = 1), and flap necrosis with underlying bone necrosis (n = 1). The authors suggested that IORT as an early boost in locally advanced resectable head and neck cancer is feasible without increasing acute toxicity.
Rutkowski et al,[46] conducting a feasibility study, evaluated the use of IORT with low-energy photons as boost in patients treated surgically for early-stage oral cancer requiring additional RT due to a high risk of local recurrence. Between 2003 and 2006, 16 patients with previously untreated early-stage (T1N0 or T2N0) oral cancer received IORT with PRS400 photon radiosurgery system (Carl Zeiss Surgical GmbH), also called INTRABEAM (IORT-PRS). All cases were SCC, and the indication for IORT and subsequent EBRT was positive surgical margin. IORT dose applied was 5, 7, or 7.5 Gy according to tumor volume and margin status. Medium follow-up time was 30 months. All patients achieved local control, while 3 patients developed regional metastases and 2 patients were diagnosed with distant metastases. Regarding complications due to IORT-PRS, a mucosal damage restricted to the tumor bed was observed in 3 cases. This way of boost delivery appeared to be feasible in a selected group of patients with early-stage oral cancer requiring postoperative RT.
Zeidan et al[47] reported their experience regarding the use of IORT for the treatment of advanced cervical metastasis from August 1982 to July 2007. A total of 231 patients were treated with surgery and IORT for advanced cervical node metastases from head and neck cancers. Indications for treatment were tumors deemed unresectable to clear margins, aggressive, large or bulky disease, or N3 nodes, suspected close or positive margins, or cases with suspected residual microscopic disease. Patients who had prior full-course EBRT were also candidates for IORT. The majority of the patients had previously undergone treatment to the neck with surgery, radiation, or both. Surgery with IORT was performed for salvage in 198 patients, and 26 patients had not been treated previously. Eighty-eight patients (39.1%) received 15 Gy or less, and 142 (60.9%) patients received more than 15 Gy. The majority of the tumors (90.9%) were SCC. Surgical margins of the neck disease were grossly or microscopically positive per frozen section in 41 patients (23.0%), close in 8 patients (4.5%), and histologically negative per frozen section in 129 patients (72.5%). Median follow-up was 1.03 years. Survival rates were 58% (1 year), 34% (3 years), and 26% (5 years). Survival was not significantly altered by margin status, dose delivered (<15 or >15 Gy), beam energy (4, 5, or 6 MeV), prior chemotherapy, or prior RT treatment. Recurrence-free survivals at 1, 3, and 5 years were 66%, 55%, and 49%, respectively. Patients treated with doses above 15 Gy had significantly improved overall relapse-free survival (P = 0.029) but not higher OS. Fifty-seven patients (25%) failed within the surgical field but only 20 patients (9%) failed within the IORT field. Local recurrence was noticed in 20 patients (9%), while 38 patients (16%) experienced regional recurrence. Distant metastases were detected in 25 patients (11%). Postoperative complications occurred in 54 patients (80 events). Among them were vascular (n = 23), pharyngocutaneous fistulas (n = 20), postoperative wound dehiscence (n = 20), neuropathies (n = 7), radiation osteonecrosis (n = 8), and necrosis of the reconstructive flaps (n = 2). The authors suggested that IORT in conjunction with EBRT might achieve better disease control in selected patients; however, further trials are necessary to determine the ideal dose and other contributing factors to minimize complications rate.
Zeidan et al[48] published their experience in the use of IORT in cancer of parotid gland. Between August 1982 and July 2007, the author's group treated 96 patients for primary or recurrent cancer of the parotid gland. Indications for treatment were tumor that could not be dissected free from vital nerves, muscles, the carotid artery, or bony structures, aggressive disease, suspected close or positive margins, or cases with suspected residual microscopic disease and previous EBRT. Thirty-three out of 96 (35.5%) had previously been irradiated. Fifty patients received 15 Gy and 39 received 20 Gy of IORT. The most common histologic subtypes were mucoepidermoid carcinoma in 20 patients, followed by SCC in 15 patients, adenoid cystic carcinoma in 11, adenocarcinoma in 10, and others. Median follow-up was 5.6 years. During this period, recurrences were presented in 32 patients that they recurred locally (n = 1), regionally (n = 19), or distally (n = 12). Recurrence-free survival rate after IORT was 82.0%, 68.5%, and 65.2% at 1, 3, and 5 years, respectively. Margin status, lymph node status, lymphovascular invasion or angiolymphatic invasion, dermal invasion, and previous chemotherapy were predictive of recurrence-free survival. Multivariate analysis showed surgery type (primary vs recurrent and tumor size to be predictive of recurrence). Overall survival rate at 1, 3, and 5 years after IORT was 88.4%, 66.1%, and 56.2%, respectively. Again, margin status, lymph node status, lymphovascular invasion or angiolymphatic invasion, dermal invasion, and previous chemotherapy were predictive of survival. Patient age was also predictive of survival. Complication rate was 27% (26 patients). There were noticed vascular complications (n = 7), trismus (n = 6), radiation osteonecroses (n = 4), fistulas (n = 4), flap necroses (n = 2), wound dehiscences (n = 2), and neuropathy (n = 1). The authors, based on their experience, stated that in selected cases of parotid cancer, adding IORT improves disease control with low recurrence rates and acceptable treatment-related complications.
Nilles-Schendera et al[54] studied 42 consecutive patients, with cancer of the floor of the mouth, from 1990 to 1996. Patients were divided into 2 groups: the first group had 28 patients with tumor staged T2-3 N0-1 and received an IORT boost dose of 12 to 15 Gy. The second group had 14 patients with small tumors, 2 to 4 cm, with invasion of the mandible, and patients with T2 tumors who were not suitable for an external irradiation. Seven out of the 28 patients of group 1 developed a local recurrence, but none primarily recurred within the IORT port. In the second group, 1 out of 14 patients experienced second tumor growth (hypopharyngeal) 6 years after primary treatment. No serious complications were reported. Survival issues were not addressed. Main advantages of IORT were greater measure of safety, in case of bridging osteosynthesis of the mandible, shorter hospital stay, and a significant gain in the quality of life in patients suffering from floor-of-mouth tumors.
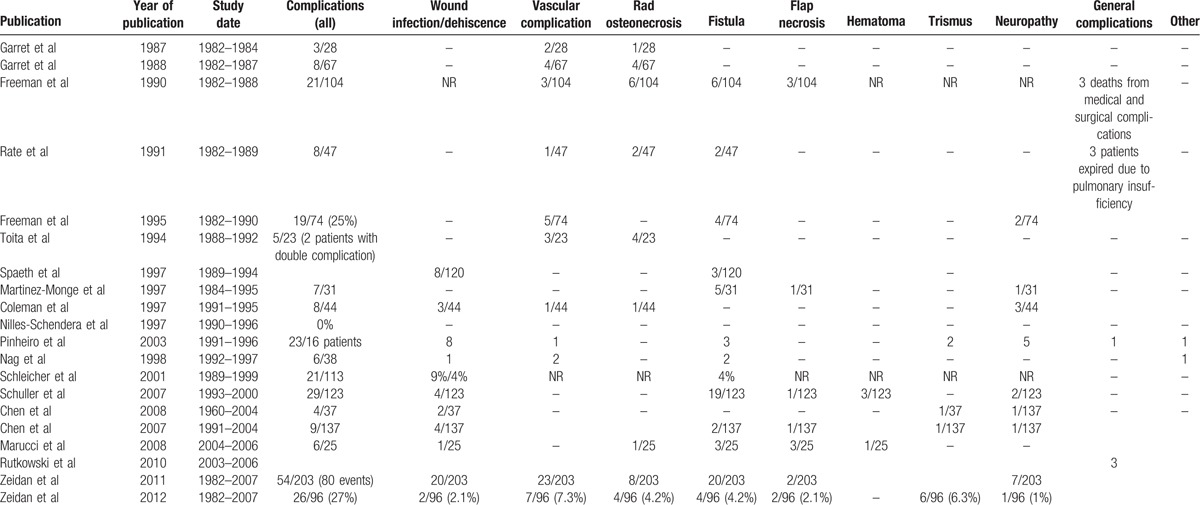
Table 1 summarizes the reported experience and outcomes of IORT use by the above mentioned investigators. In Table 2, the complication rates of IORT usage are summarized.
Table 2.
Complications of intraoperative radiotherapy (IORT).
4. Discussion
Treatment of advanced and recurrent cancer in head and neck region comprises a major therapeutic challenge. Surgical resection of recurrences, if it is feasible, is considered the best option for disease local control.[49–50] Many investigators believe that the results of surgery can be improved with additional therapy such as EBRT, IORT, and chemotherapy. Postresection EBRT seems to improve locoregional control but with the drawback of increased toxicity since tissues surrounding the target were exposed to high doses of radiation when primary treatment was delivered.[51–52]
IORT seems able to overcome this limitation of EBRT. By using IORT in head and neck cancer, we are able to deliver a high dose of electron beam energy directly to the target region, which is biologically equivalent to 2 to 3 times the same dose delivered via EBRT.[24] With this modality radiation energy to the surrounding structures including neurovascular and bony structures, except for the suture line, anastomosis is also kept in minimum levels. This is further achieved by displacing them with retraction and packing or protecting them with strategically placed shields. Moreover, radiation delivery at the time of definitive resection is particularly important in head and neck cancer, where the total treatment package time from the day of surgery to the end of radiation therapy is crucial for locoregional control and survival optimization. Improved survival and locoregional control when patients with advanced head and neck cancer received radiation within 11 weeks postoperatively has been reported.[3,13] It seems that the integration of IORT in multidisciplinary treatment of primary cancers offers the chance to decrease total treatment package time. Furthermore, the delivery of a large dose of radiation could presumably overcome radioresistant tumor clonogens that have been refractory to a previous EBRT.[21–24]
In the studies that we reviewed, main indications for IORT were advanced head and neck disease deemed poorly resectable or unresectable and recurrent head and neck neoplastic disease that had been previously treated with other modalities. In the majority of cases, histologic diagnosis was SCC followed by salivary gland malignancies. Delivered IORT dose ranged from 7.5 to 30 Gy, and median was 20 Gy. However, in most contemporary studies, a trend to lower the delivered doses trying to reduce toxicity and complications was noticed. Reported local control rates with the addition of IORT modality appear as high as 90% in a 2-year follow-up in selected cases where no residual disease is noticed after surgical excision.[21,26–29,31–39,42,46–48] The combination of EBRT postoperatively seems to further improve local control.[31,36,53,54] Furthermore, the length of hospital stay is not appreciably prolonged when IOERT is used as a treatment adjunct to surgery.[55]
A benefit of the 2-year DFS has been reported as well. However, long-term survival rates do not seem to conform in all series.[26,31,33,34,36–38]
In most of the studies, gross residual disease is a constant factor predicting a poor patient outcome. Local failure rates increase when histologic margins at the time of salvage surgery shift from negative to microscopic, reaching 100% in cases of gross residual disease.[27,29–35,37,47,48] It seems that patients with advanced disease, especially with carotid involvement, have the most dismal median OS of 1 year accompanied by high complication rates of 50%. This group of patients is at high risk for post-treatment cerebrovascular events and neurologic sequelae.[56]
Thus, it seems that patients who receive the greatest benefit from IORT are those who have no microscopic residual disease, or preferably only close surgical margins by conventional light microscopic criteria. Nevertheless, even patients with gross residual disease may gain some short-term pain relief from the addition of IORT following subtotal resection despite high rates of locoregional failure.[26] A good palliative effect has been obtained in patients treated for extensive recurrence in previously irradiated fields.[32–34,57,58]
Notwithstanding, determining the potential benefit of IORT in multimodality treatment of advanced and recurrent head and neck cancer is not safe based on the published studies so far. Conflicting results are reported, and the studies are mainly retrospective and cannot be compared directly. Several issues complicate the interpretation of these studies such as the diversity of the treated population presenting with different histologic diagnoses and different stages of the disease that have been differently treated previously. Patients suffered either from recurrent or primary disease and also many of them had been treated with EBRT before. For example, unresectable patients are at high risk of failure at the primary or the neck and for metastatic disease. The result of IORT in them could have been different compared to patients with less-advanced disease. Other patients had been treated in the primary site and others in the neck. Moreover, the variety of adjuvant and neoadjuvant modalities cannot determine the benefit attributed to IORT. An interesting finding is that the reported local control and survival rates are higher for salivary glands malignant tumors compared to SCC.[26,28,37,48] This difference probably reflects the different natural history of these 2 diseases rather than a greater radiosensitivity of non-SCC tumors.
The morbidity of IORT is summarized in Table 2. When reviewing the various clinical experiences with IORT, one must balance the potential of IORT-related morbidity against the possibility of tumor recurrence/persistence resulting in tumor-related complications. As mentioned above, the median delivered dose of IORT has been reduced in the latest reported series aiming at reducing toxicity and complications. IORT at 14 to 20 Gy appears to be safe in the majority of cases with a complication rate around 20%. The overwhelming majority consisted of minor complications such as wound dehiscence and/or delayed healing. Less-common toxicities included motosensory deficits, neuropathic pain, dysphagia, xerostomia, fistula formation, osteoradionecrosis, flap necrosis, and trismus. The majority of these complications are thought to reflect the scope of the surgery in general for patients with advanced disease. Many of them suffered from recurrent or persistent cancer after prior surgery, RT, and chemotherapy. Considering the fact that many patients had been reirradiated or had undergone previous chemotherapy, the surgical complication rate in such population would have been high regardless of IORT treatment. Fatal complications such as carotid occlusion or rupture only seldom occurred and appear to be related to doses >20 Gy.[27] Some investigators argue that the use of myocutaneous flap reconstruction at the time of IORT might increase the therapeutic index in cases of recurrent head and neck cancer. By replacing previously irradiated tissue with a healthy well vascularized radiation-naïve flap, a good healing and functional outcome could be achieved in majority of patients permitting the use of further postoperative EBRT after sufficient wound healing.[40]
Clear conclusions about IORT are difficult to produce. Most studies are pilot studies with relatively small samples of patients with varying degrees of surgical resection, mixed stages, varying radiation doses, and other factors that may influence the outcome. The benefit and long-term efficacy of IORT can only be established by properly designed randomized clinical trials. IORT usage in the treatment of head and neck cancer is rather limited, partially due to difficulties in administering, although several studies have reported promising results of its integration in multimodality treatment. It seems that IOERT is best suited for patients with recurrent head and neck cancer who are able to undergo surgical resection without macroscopic residual disease. Doses of 7.5 to 20 Gy are recommended based on margin status and previous irradiation treatment. Preliminary studies have demonstrated the safety of IORT delivery in treating patients with locally advanced head and neck cancer, in the context of aggressive combined modality therapy. IORT is generally well tolerated without significantly increasing the complications rate but it is seems important to incorporate reconstructive surgery to restore form and function as well as to maximize tolerance to adjuvant therapy. In addition, for symptomatic patients who have undergone a subtotal resection, IOERT as a boost seems to be a reasonable palliative approach if it is available.
In head and neck malignancies, distant and disseminating disease is probably the dominant issue. As newer agents arise regarding treatment of systematic disease, locoregional control still remains a major issue since it is accompanied by major morbidity and mortality. The promising preliminary results necessitate exploring the use of IORT in patients with earlier stage disease and its integration with EBRT in order to substantially decrease recurrence and improve survival by optimizing locoregional control of upper aerodigestive tract cancer. This could be further achieved by defining accurate criteria for patient selection that could have the most benefit from this modality. Given that patients with recurrent head and neck cancer have doom outcomes, with salvage surgery providing durable disease control in approximately 15% of such patients, and the addition of EBRT in another 5%, IORT might have a role in the multidisciplinary management of the disease. IORT seems to confer optimized local control rates, as well as OS. Notwithstanding, aggressive surgery, EBRT, and chemotherapy are the mainstays of therapy in this particular group.
Footnotes
Abbreviations: DFS = disease-free survival, EBRT = external-beam radiotherapy, Gy = Gray, IOERT = intraoperative electron-beam radiotherapy, IORT = intraoperative radiotherapy, IORT-PRS = IORT with PRS400 photon radiosurgery system (Carl Zeiss Surgical GmbH), also called INTRABEAM, OS = overall survival, SCC = squamous cell carcinoma.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29. [DOI] [PubMed] [Google Scholar]
- [2].Carter YM, Jablons DM, DuBois JB, et al. Intraoperative radiation therapy in the multimodality approach to upper aerodigestive tract cancer. Surg Oncol Clin N Am 2003;12:1043–63. [DOI] [PubMed] [Google Scholar]
- [3].Ang KK, Trotti A, Brown BW, et al. Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys 2001;51:571–8. [DOI] [PubMed] [Google Scholar]
- [4].Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 2004;350:1945–52. [DOI] [PubMed] [Google Scholar]
- [5].Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 2004;350:1937–44. [DOI] [PubMed] [Google Scholar]
- [6].Scala LM, Hu K, Urken ML, et al. Intraoperative high-dose-rate radiotherapy in the management of locoregionally recurrent head and neck cancer. Head Neck 2013;35:485–92. [DOI] [PubMed] [Google Scholar]
- [7].Popovtzer A, Gluck I, Chepeha DB, et al. The pattern of failure after reirradiation of recurrent squamous cell head and neck cancer: implications for defining the targets. Int J Radiat Oncol Biol Phys 2009;74:1342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Perry DJ, Chan K, Wolden S, et al. High-dose-rate intraoperative radiation therapy for recurrent head-and-neck cancer. Int J Radiat Oncol Biol Phys 2010;76:1140–6. [DOI] [PubMed] [Google Scholar]
- [9].Hanna E, Alexiou M, Morgan J, et al. Intensive chemoradiotherapy as a primary treatment for organ preservation in patients with advanced cancer of the head and neck: efficacy, toxic effects, and limitations. Arch Otolaryngol Head Neck Surg 2004;130:861–7. [DOI] [PubMed] [Google Scholar]
- [10].Fu KK. Combined-modality therapy for head and neck cancer. Oncology (Williston Park) 1997;11:1781–90. 1796; discussion 1796, 1179. [PubMed] [Google Scholar]
- [11].Al-Sarraf M, Pajak TF, Byhardt RW, et al. Postoperative radiotherapy with concurrent cisplatin appears to improve locoregional control of advanced, resectable head and neck cancers: RTOG 88-24. Int J Radiat Oncol Biol Phys 1997;37:777–82. [DOI] [PubMed] [Google Scholar]
- [12].Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol 1998;16:1310–7. [DOI] [PubMed] [Google Scholar]
- [13].Rosenthal DI, Liu L, Lee JH, et al. Importance of the treatment package time in surgery and postoperative radiation therapy for squamous carcinoma of the head and neck. Head Neck 2002;24:115–26. [DOI] [PubMed] [Google Scholar]
- [14].De Crevoisier R, Bourhis J, Domenge C, et al. Full-dose reirradiation for unresectable head and neck carcinoma: experience at the Gustave-Roussy Institute in a series of 169 patients. J Clin Oncol 1998;16:3556–62. [DOI] [PubMed] [Google Scholar]
- [15].Mouttet-Audouard R, Gras L, Comet B, et al. Reirradiation for recurrent or second primary head and neck cancers. Bull Cancer 2011;98:1477–88. [DOI] [PubMed] [Google Scholar]
- [16].Mouttet-Audouard R, Gras L, Comet B, et al. Evidence based and new developments in re-irradiation for recurrent or second primary head and neck cancers. Curr Opin Otolaryngol Head Neck Surg 2012;20:137–41. [DOI] [PubMed] [Google Scholar]
- [17].Abe M, Shibamoto Y, Ono K, et al. Intraoperative radiation therapy for carcinoma of the stomach and pancreas. Front Radiat Ther Oncol 1991;25:258–69. discussion 330–253. [DOI] [PubMed] [Google Scholar]
- [18].Abe M, Takahashi M. Intraoperative radiotherapy: the Japanese experience. Int J Radiat Oncol Biol Phys 1981;7:863–8. [DOI] [PubMed] [Google Scholar]
- [19].Hu K, Yom S, Kaplan M. Gunderson LL, Willett CG, Calvo FA, Harrison LB, et al. Head and neck cancer. Intraoperative Irradiation: Techniques and Results. Current Clinical Oncology 2nd edn.New York, NY:Springer Science Business Media; 2011. 163–88. [Google Scholar]
- [20].Debenham BJ, Hu KS, Harrison LB. Present status and future directions of intraoperative radiotherapy. Lancet Oncol 2013;14:457–64. [DOI] [PubMed] [Google Scholar]
- [21].Marucci L, Pichi B, Iaccarino G, et al. Intraoperative radiation therapy as an “early boost” in locally advanced head and neck cancer: preliminary results of a feasibility study. Head Neck 2008;30:701–8. [DOI] [PubMed] [Google Scholar]
- [22].Calvo FA, Meirino RM, Orecchia S R. Intraoperative radiation therapy part 2. Clinical results. Crit Rev Oncol Hematol 2006;59:116–27. [DOI] [PubMed] [Google Scholar]
- [23].Calvo FA, Meirino RM, Orecchia R. Intraoperative radiation therapy first part: rationale and techniques. Crit Rev Oncol Hematol 2006;59:106–15. [DOI] [PubMed] [Google Scholar]
- [24].Willett CG, Czito BG, Tyler DS. Intraoperative radiation therapy. J Clin Oncol 2007;25:971–7. [DOI] [PubMed] [Google Scholar]
- [25].Dale RG. The application of the linear-quadratic dose-effect equation to fractionated and protracted radiotherapy. Br J Radiol 1985;58:515–28. [DOI] [PubMed] [Google Scholar]
- [26].Chen AM, Garcia J, Bucci MK, et al. Recurrent salivary gland carcinomas treated by surgery with or without intraoperative radiation therapy. Head Neck 2008;30:2–9. [DOI] [PubMed] [Google Scholar]
- [27].Garrett P, Pugh N, Ross D, et al. Intraoperative radiation therapy for advanced or recurrent head and neck cancer. Int J Radiat Oncol Biol Phys 1987;13:785–8. [DOI] [PubMed] [Google Scholar]
- [28].Freeman SB, Hamaker RC, Singer MI, et al. Intraoperative radiotherapy of head and neck cancer. Arch Otolaryngol Head Neck Surg 1990;116:165–8. [DOI] [PubMed] [Google Scholar]
- [29].Rate WR, Garrett P, Hamaker R, et al. Intraoperative radiation therapy for recurrent head and neck cancer. Cancer 1991;67:2738–40. [DOI] [PubMed] [Google Scholar]
- [30].Freeman SB, Hamaker RC, Rate WR, et al. Management of advanced cervical metastasis using intraoperative radiotherapy. Laryngoscope 1995;105:575–8. [DOI] [PubMed] [Google Scholar]
- [31].Martinez-Monge R, Azinovic I, Alcalde J, et al. IORT in the management of locally advanced or recurrent head and neck cancer. Front Radiat Ther Oncol 1997;31:122–5. [DOI] [PubMed] [Google Scholar]
- [32].Toita T, Nakano M, Takizawa Y, et al. Intraoperative radiation therapy (IORT) for head and neck cancer. Int J Radiat Oncol Biol Phys 1994;30:1219–24. [DOI] [PubMed] [Google Scholar]
- [33].Spaeth J, Andreopoulos D, Unger T, et al. Intra-operative radiotherapy – 5 years of experience in the palliative treatment of recurrent and advanced head and neck cancers. Oncology 1997;54:208–13. [DOI] [PubMed] [Google Scholar]
- [34].Schleicher UM, Phonias C, Spaeth J, et al. Intraoperative radiotherapy for pre-irradiated head and neck cancer. Radiother Oncol 2001;58:77–81. [DOI] [PubMed] [Google Scholar]
- [35].Chen AM, Bucci MK, Singer MI, et al. Intraoperative radiation therapy for recurrent head-and-neck cancer: the UCSF experience. Int J Radiat Oncol Biol Phys 2007;67:122–9. [DOI] [PubMed] [Google Scholar]
- [36].Coleman CW, Roach M, 3rd, Ling SM, et al. Adjuvant electron-beam IORT in high-risk head and neck cancer patients. Front Radiat Ther Oncol 1997;31:105–11. [DOI] [PubMed] [Google Scholar]
- [37].Pinheiro AD, Foote RL, McCaffrey TV, et al. Intraoperative radiotherapy for head and neck and skull base cancer. Head Neck 2003;25:217–25. discussion 225-216. [DOI] [PubMed] [Google Scholar]
- [38].Nag S, Schuller DE, Martinez-Monge R, et al. Intraoperative electron beam radiotherapy for previously irradiated advanced head and neck malignancies. Int J Radiat Oncol Biol Phys 1998;42:1085–9. [DOI] [PubMed] [Google Scholar]
- [39].Grecula JC, Schuller DE, Smith R, et al. Long-term follow-up on an intensified treatment regimen for advanced resectable head and neck squamous cell carcinomas. Cancer Invest 2001;19:127–36. [DOI] [PubMed] [Google Scholar]
- [40].Most MD, Allori AC, Hu K, et al. Feasibility of flap reconstruction in conjunction with intraoperative radiation therapy for advanced and recurrent head and neck cancer. Laryngoscope 2008;118:69–74. [DOI] [PubMed] [Google Scholar]
- [41].Ozer E, Grecula JC, Agrawal A, et al. Long-term results of a multimodal intensification regimen for previously untreated advanced resectable squamous cell cancer of the oral cavity, oropharynx, or hypopharynx. Laryngoscope 2006;116:607–12. [DOI] [PubMed] [Google Scholar]
- [42].Schuller DE, Ozer E, Agrawal A, et al. Multimodal intensification regimens for advanced, resectable, previously untreated squamous cell cancer of the oral cavity, oropharynx, or hypopharynx: a 12-year experience. Arch Otolaryngol Head Neck Surg 2007;133:320–6. [DOI] [PubMed] [Google Scholar]
- [43].Schuller DE, Grecula JC, Gahbauer RA, et al. Intensified regimen for advanced head and neck squamous cell carcinomas. Arch Otolaryngol Head Neck Surg 1997;123:139–44. [DOI] [PubMed] [Google Scholar]
- [44].Schuller DE, Grecula JC, Agrawal A, et al. Multimodal intensification therapy for previously untreated advanced resectable squamous cell carcinoma of the oral cavity, oropharynx, or hypopharynx. Cancer 2002;94:3169–78. [DOI] [PubMed] [Google Scholar]
- [45].Grecula JC, Schuller DE, Rhoades CA, et al. Intensification regimen 2 for advanced head and neck squamous cell carcinomas. Arch Otolaryngol Head Neck Surg 1999;125:1313–8. [DOI] [PubMed] [Google Scholar]
- [46].Rutkowski T, Wygoda A, Hutnik M, et al. Intraoperative radiotherapy (IORT) with low-energy photons as a boost in patients with early-stage oral cancer with the indications for postoperative radiotherapy: treatment feasibility and preliminary results. Strahlenther Onkol 2010;186:496–501. [DOI] [PubMed] [Google Scholar]
- [47].Zeidan YH, Yeh A, Weed D, et al. Intraoperative radiation therapy for advanced cervical metastasis: a single institution experience. Radiat Oncol 2011;6:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zeidan YH, Shiue K, Weed D, et al. Intraoperative radiotherapy for parotid cancer: a single-institution experience. Int J Radiat Oncol Biol Phys 2012;82:1831–6. [DOI] [PubMed] [Google Scholar]
- [49].Parsons JT, Mendenhall WM, Stringer SP, et al. Salvage surgery following radiation failure in squamous cell carcinoma of the supraglottic larynx. Int J Radiat Oncol Biol Phys 1995;32:605–9. [DOI] [PubMed] [Google Scholar]
- [50].Goodwin WJ., Jr Salvage surgery for patients with recurrent squamous cell carcinoma of the upper aerodigestive tract: when do the ends justify the means? Laryngoscope 2000;110(3 pt 2 suppl 93):1–8. [DOI] [PubMed] [Google Scholar]
- [51].Janot F, de Raucourt D, Benhamou E, et al. Randomized trial of postoperative reirradiation combined with chemotherapy after salvage surgery compared with salvage surgery alone in head and neck carcinoma. J Clin Oncol 2008;26:5518–23. [DOI] [PubMed] [Google Scholar]
- [52].Kasperts N, Slotman BJ, Leemans CR, et al. Results of postoperative reirradiation for recurrent or second primary head and neck carcinoma. Cancer 2006;106:1536–47. [DOI] [PubMed] [Google Scholar]
- [53].Schmitt T, Prades JM, Favrel V, et al. IORT for locally advanced oropharyngeal carcinomas with major extension to the base of the tongue: 5-year results of a prospective study. Front Radiat Ther Oncol 1997;31:117–21. [DOI] [PubMed] [Google Scholar]
- [54].Nilles-Schendera A, Bruggmoser G, Stoll P, et al. IORT in floor of the mouth cancer. Front Radiat Ther Oncol 1997;31:102–4. [DOI] [PubMed] [Google Scholar]
- [55].Haller JR, Mountain RE, Schuller DE, et al. Mortality and morbidity with intraoperative radiotherapy for head and neck cancer. Am J Otolaryngol 1996;17:308–10. [DOI] [PubMed] [Google Scholar]
- [56].Freeman SB, Hamaker RC, Borrowdale RB, et al. Management of neck metastasis with carotid artery involvement. Laryngoscope 2004;114:20–4. [DOI] [PubMed] [Google Scholar]
- [57].Ravasz LA, Hordijk GJ, Slootweg PJ, et al. Uni- and multivariate analysis of eight indications for post-operative radiotherapy and their significance for local-regional cure in advanced head and neck cancer. J Laryngol Otol 1993;107:437–40. [DOI] [PubMed] [Google Scholar]
- [58].Freeman SB. Advanced cervical metastasis involving the carotid artery. Curr Opin Otolaryngol Head Neck Surg 2005;13:107–11. [DOI] [PubMed] [Google Scholar]


