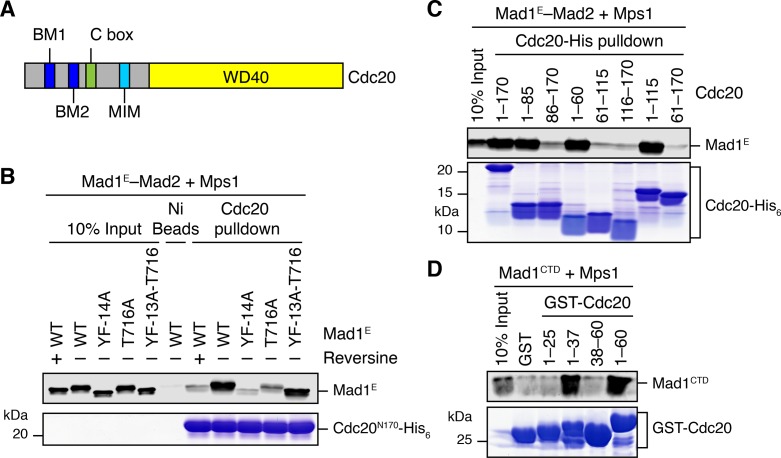
Figure 6. Phosphorylation of Mad1 T716 promotes its binding to Cdc20.
(A) Domains and motifs of Cdc20. C box, a conserved APC/C-binding motif; MIM, Mad2-interacting motif; BM1, basic motif 1 (27RWQRK31); BM2, basic motif 2 (54RTPGRTPGK62). (B) In vitro pull-down of the indicated Mad1E–Mad2 complexes (which had been pre-treated with the kinase domain of Mps1) by Ni2+ beads bound to Cdc20N170-His6. The bait protein was stained with Coomassie, and the prey proteins bound to beads were blotted with the anti-Mad1 antibody. (C) In vitro pull-down of the Mad1E–Mad2 complex (which had been pre-treated with the kinase domain of Mps1) by Ni2+ beads bound to the indicated Cdc20-His6 proteins. The bait proteins were stained with Coomassie, and the prey proteins bound to beads were blotted with the anti-Mad1 antibody. (D) In vitro pull-down of Mad1CTD (which had been pre-treated with the kinase domain of Mps1) by beads bound to the indicated GST-Cdc20 fragments. The bait proteins were stained with Coomassie, and the prey proteins bound to beads were blotted with the anti-Mad1 antibody.