Figure 2.
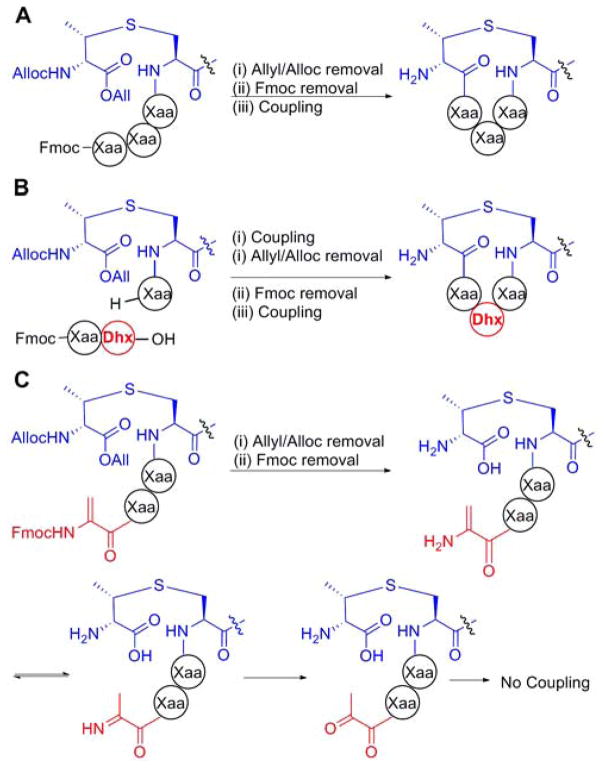
(A) Orthogonal protecting groups on a (methyl)lanthionine building block (DL-MeLan here) allow elongation and subsequent cyclization of a peptide. For alternative protecting group schemes, see 10 (B) Introduction of short oligopeptides containing dehydro amino acids (Dhx). (C) If the amine coupling partner for cyclization is a dehydro amino acid (Dha here), the low reactivity of the enamine promotes hydrolysis to the ketone preventing cyclization.