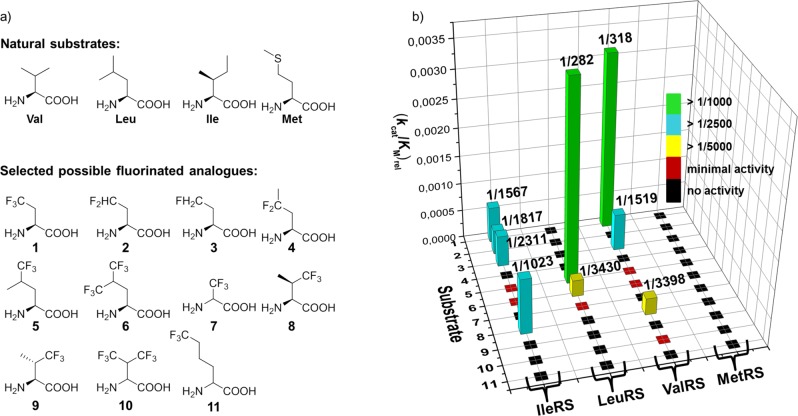
Figure 2.
(a) Chemical structure of natural substrates of class Ia AARSs and putative fluorinated analogues. 1: (2S)-4,4,4-trifluoroethylglycine (TfeGly), 2: (2S)-4,4-difluoroethylglycine (DfeGly), 3: (2S)-4-monofluoroethylglycine (MfeGly), 4: (2S)-4,4-difluoropropylglycine (DfpGly), 5: (2S,4S,4R)-5,5,5-trifluoroleucine (TfLeu), 6: (2S)-5,5,5,5′,5′,5′-hexafluoroleucine (HfLeu), 7: (2S,2R)-3,3,3-trifluoroalanine (TfAla), 8: (2S,3R)-4,4,4-trifluorovaline ((2S,3R)-TfVal), 9: (2S,3S)-4,4,4-trifluorovaline ((2S,3S)-TfVal), 10: (2S,2R)-4,4,4,4′,4′,4′-hexafluorovaline (HfVal), 11: (2S,2R)-6,6,6-trifluoronorleucine (TfNLeu), (b) Kinetic data that were determined in this study for the activation of these fluorinated AAs by the indicated class Ia AARSs.