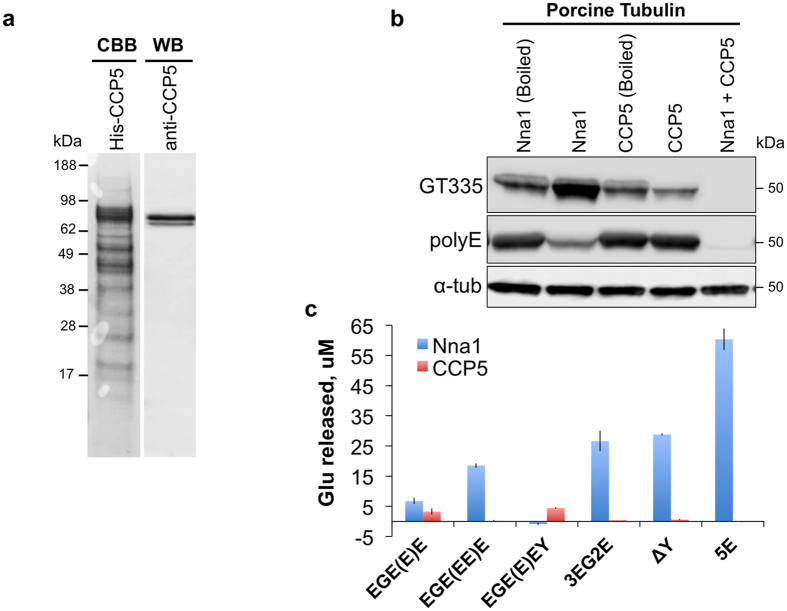
Figure 2. Purified recombinant CCP5 catalyzes the deglutamylation of porcine tubulin and synthetic substrates.
(a) SDS-PAGE of purified recombinant CCP5 (DQ867034 splice variant) stained with Coomassie brilliant blue (CBB) (left lane). The major CBB band is immunoreactive with a CCP5 specific antibody (right lane). (b) Recombinant CCP5 and/or Nna1 were incubated with porcine tubulin and the deglutamylation activity was monitored by immunoblotting using GT335 and polyE antibodies. CCP5, but not the heat-denatured (Boiled) enzyme, reduced the GT335 signal without altering polyE immunoreactivity, indicative of specific removal of the branching glutamate of tubulin. Nna1 alone substantially reduced polyE immunoreactivity, though it increased the GT335 signal. Co-incubation of Nna1 and CCP5 completely abolished GT335 signal and further reduced polyE signal. (c) CCP5 is not active against three Nna1 synthetic substrates (Biotin-3EG2E, Biotin-ΔY, and Biotin-5E4), but it is active against a substrate with an exposed γ-carboxyl-linked glutamate (Biotin-EGE(E)E). When the γ-carboxyl-linked glutamate is in chain with another α-linked glutamate (Biotin-EGE(EE)E), CCP5 is no longer active. CCP5, but not Nna1, is active against Biotin-EGE(E)EY, where the only terminal glutamate is linked through a γ-carboxyl, further confirming their substrate specificities. Bars are mean ± SEM (error bars) of triplicate determinations.