Figure 3.
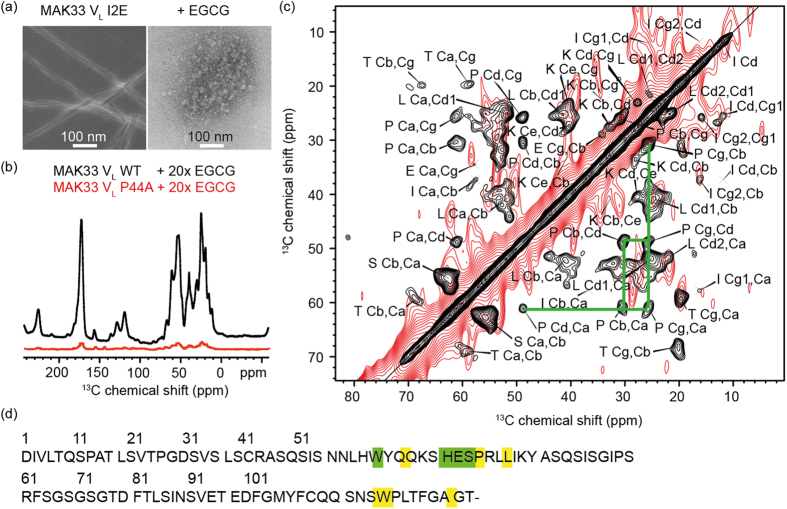
(a) Precipitates of MAK33 VL I2E visualised by TEM. To enable use of native-like conditions for fibril formation, the highly amyloidogenic I2E mutant was employed. Fibrils were grown by incubating a 50 μM protein solution at a temperature of 37 °C and agitating the sample at 120 rpm (left). The sample with EGCG-induced precipitates (right) was treated identically, but in the presence of a 10-fold excess of EGCG. (b) MAS solid-state NMR analysis of coprecipitates of EGCG with the WT or the P44A mutant. The P44A precipitates yield a drastically reduced signal intensity in 1H,13C-cross polarization experiments. (c) 2D 13C,13C PDSD correlation spectrum of coprecipitates of MAK33 VL WT and EGCG (black). Nine spin systems could be identified, which presumably originate from residues close to the EGCG binding site. In the lower right part of the symmetric spectrum, the proline spin system is marked with green lines. The NMR spectrum of the P44A variant prepared in the same way (red) does not yield a comparable cross peak pattern. (d) Assigned spin systems of EGCG-induced WT-VL aggregates. The identified spin systems were assigned to those residues, which are closest to the EGCG binding site in the unguided docking structure (Fig. 2b; details in SI 4). Unambiguous assignments are shown in green, ambiguous assignments in yellow.