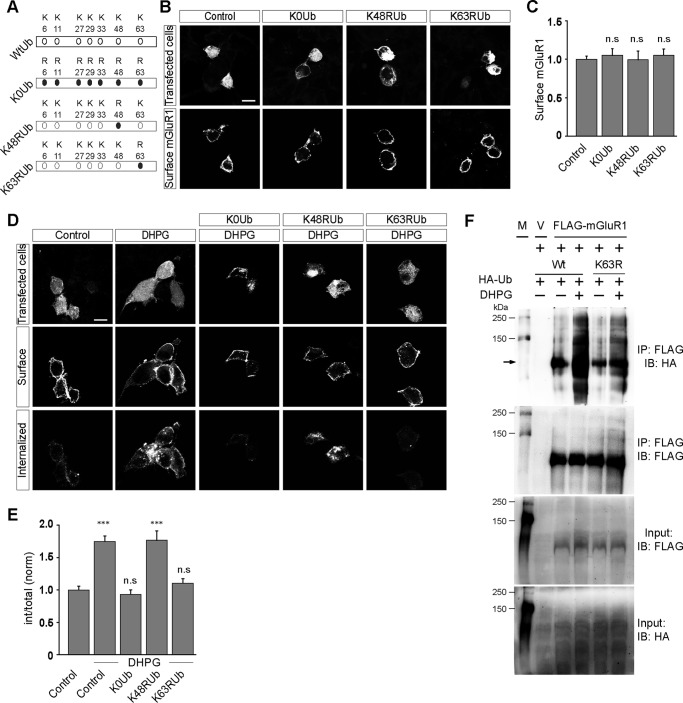
FIGURE 3.
Endocytosis of mGluR1 is Lys63-linked polyubiquitination-dependent. A, schematic showing the ubiquitin constructs (K0Ub, K48Rub, and K63RUb) used for this study. B, surface expression of myc-mGluR1 upon overexpression of various ubiquitin constructs as shown in representative images. C, quantitation shows that overexpression of K0Ub, K48Rub, and K63RUb did not have any effect on the surface expression of the receptor. D and E, however, overexpression of both K0Ub and K63RUb inhibited the DHPG-mediated internalization of myc-mGluR1 as shown by the representative images (D) and quantitation (E). On the other hand, K48RUb overexpression had no effect on the endocytosis of the receptor. F, co-immunoprecipitation assays demonstrate that application of R,S-DHPG resulted in the incorporation of HA-K63RUb in mGluR1. M, molecular weight markers; V, empty vector; IB, immunoblot; IP, immunoprecipitation. All the results are represented as means ± S.E., collected from three independent experiments. Scale bar, 10 μm. ***, p < 0.001; n.s, p > 0.05.