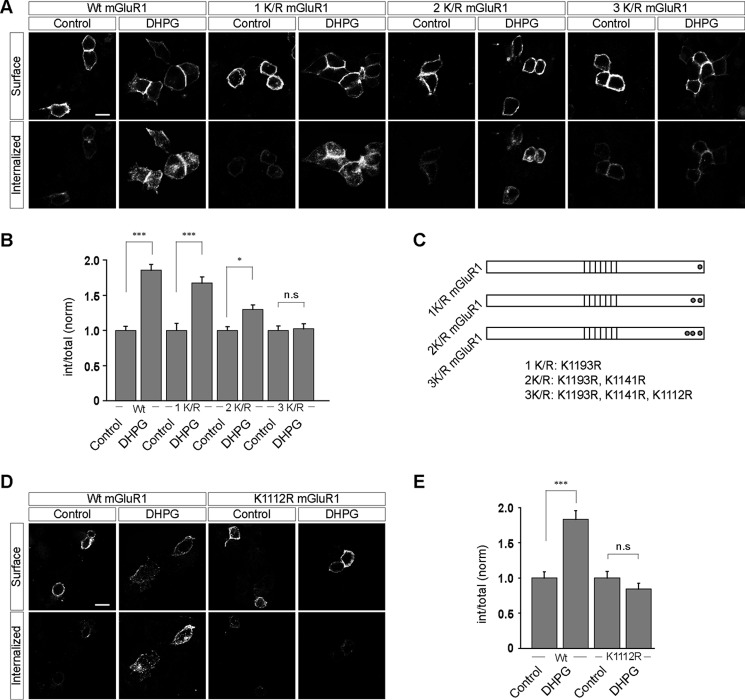
FIGURE 4.
Lys1112 residue of mGluR1 plays critical role in the ubiquitin-dependent internalization of the receptor in HEK293 cells. A, representative images showing the internalization of various mutants of mGluR1 (1K/R, 2K/R, and 3K/R) on application of 100 μm R,S-DHPG in HEK293 cells. B, quantitation suggested that myc-mGluR1 1K/R internalized normally like the wild type receptor, whereas myc-mGluR1 2K/R showed lesser internalization on application of the ligand. Finally, myc-mGluR1 3K/R failed to internalize on application of 100 μm R,S-DHPG. C, schematic showing the position of various mutations in the 1K/R, 2K/R, and 3K/R constructs. D, mutation of Lys1112 residue of mGluR1 to arginine alone completely inhibited the ligand-mediated endocytosis of the receptor as shown in the representative images (D) and quantitation (E). All the results are represented as means ± S.E., collected from three independent experiments. Scale bar, 10 μm. ***, p < 0.001; *, p < 0.05; n.s, p > 0.05.