FIGURE 5.
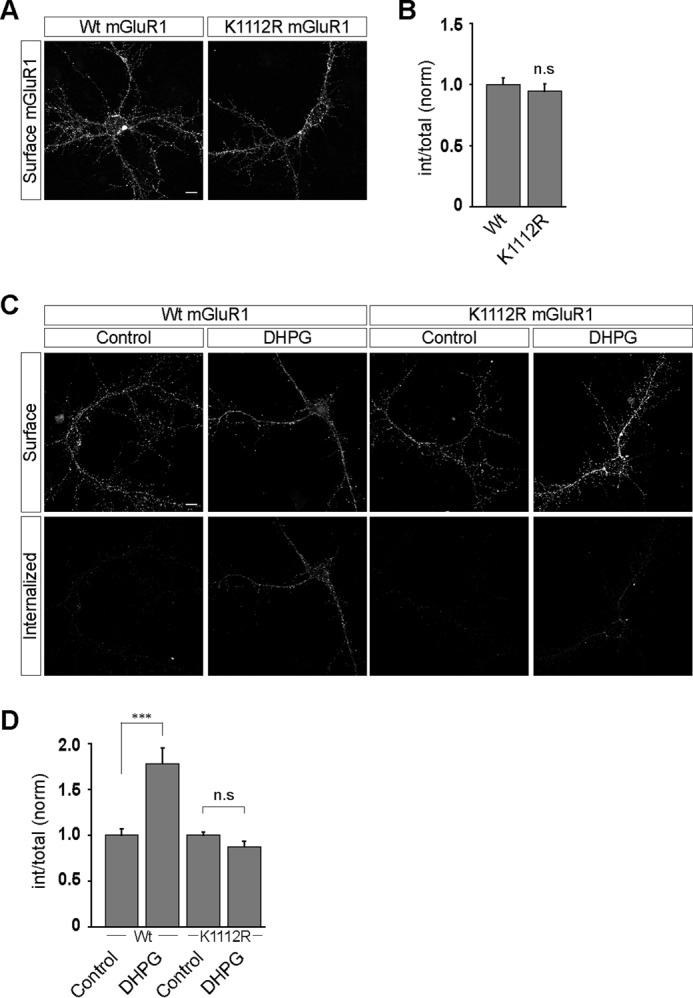
Mutation of lysine 1112 residue to arginine in mGluR1 blocks the ligand-mediated endocytosis of the receptor in primary hippocampal neurons. A, mutating the lysine 1112 residue at the C-terminal tail of mGluR1 to arginine had no effect on the surface expression of the receptor in the primary hippocampal neurons as shown in representative images. B, quantitation also suggested that both wild type and K1112R myc-mGluR1 manifested similar level of expression at the cell surface. C, representative images showing that although wild type myc-mGluR1 internalized normally on R,S-DHPG application, K1112R myc-mGluR1 did not internalize on ligand application. D, quantitation also suggested that mutation of Lys1112 to Arg in mGluR1 resulted in the complete blocking of the DHPG-mediated internalization of the receptor. All the results are represented as means ± S.E., collected from three independent experiments. Scale bar, 10 μm. ***, p < 0.001; n.s, p > 0.05.
