FIGURE 6.
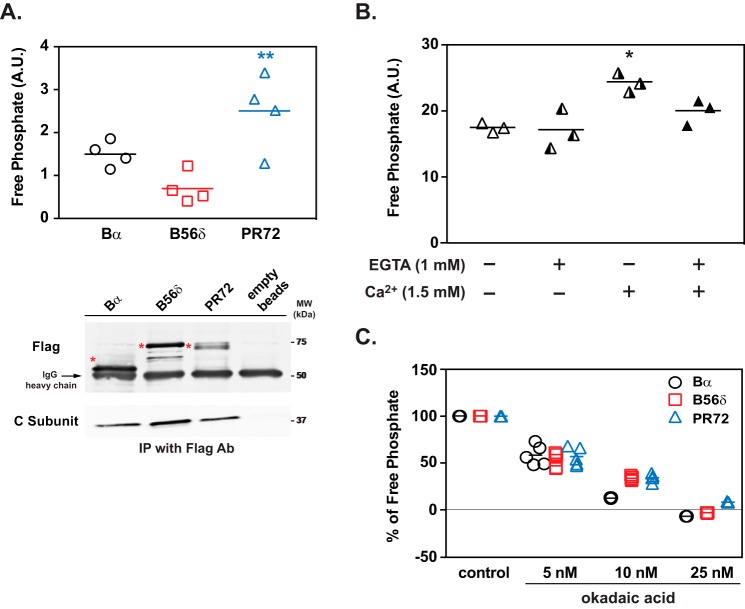
Dephosphorylation of DARPP-32 at Ser-97 by PP2A/PR72. A, in vitro phosphatase assay of PP2A heterotrimers containing either Bα, B56δ, or PR72 and using [32P]Ser(P)-97-DARPP-32 as a substrate. FLAG-tagged Bα, B56δ, and PR72, immunoprecipitated as heterotrimers from lysates of HEK293 cells overexpressing individual tagged B subunits, were used for the PP2A assay. The activity of PP2A heterotrimers was normalized to the amount of the C subunit, which was measured by immunoblotting (lower panels). The positions of the B subunits are indicated with asterisks. Data for four experiments done in triplicate are presented in scatter plots with the mean values indicated. **, p < 0.01 compared with PP2A/B56δ, one-way ANOVA followed by Tukey's test. B, in vitro analysis of Ca2+-dependent regulation of PP2A/PR72. Activity of PP2A-PR72 against [32P]Ser(P)-97-DARPP-32 was measured in the absence or presence of Ca2+ (1.5 mm) and/or EGTA (1 mm). The absolute values of PP2A/PR72 activity were different from panel A due to different conditions of [32P]Ser(P)-97-DARPP-32 and C-subunit normalization, but the values in panels A or B are uniform and comparable among treatment conditions. Data for three experiments done in triplicate are presented in scatter plots with the mean values indicated. *, p < 0.05 compared with untreated condition and EGTA alone, one-way ANOVA followed by Tukey's test. C, sensitivity of PP2A/regulatory subunit complexes to okadaic acid in vitro using [32P]Ser(P)-97-DARPP-32 as a substrate. The ability of okadaic acid to inhibit the activity of different PP2A heterotrimers containing Bα, B56δ, or PR72 (isolated as described above) was measured. The activity of PP2A heterotrimers was normalized to the amount of C subunit for each complex, which was measured by immunoblotting. Data for five experiments done in triplicate are presented in scatter plots with the mean values indicated. A.U., arbitrary units.