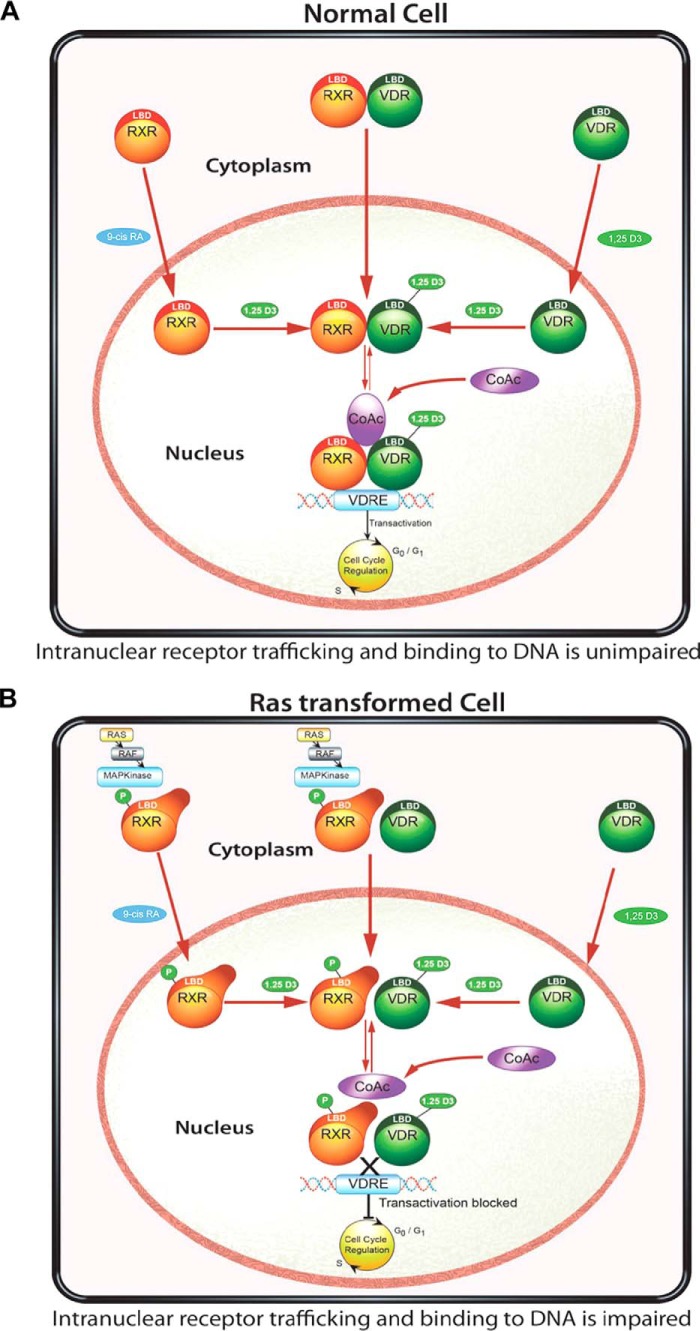
FIGURE 9.
Proposed model for nuclear import of VDR, RXR, and VDR/RXR interaction and DNA binding in non-transformed and Ras-transformed cells. In normal cells (A), the nuclear import of VDR and RXR is mediated by their respective ligands. Once in the nucleus, 1,25(OH)2D3 binding to VDR is critical for the VDR-RXR heterodimer interaction and binding to the hormone-response elements, recruitment of co-factors (CoAc), and effect on 1,25(OH)2D3 signaling. In the Ras-transformed keratinocyte (B), phosphorylation of RXR prevents the nuclear translocation of RXR and binding of the VDR-RXR complex to the hormone-response element. The recruitment of co-factors was impaired thus preventing 1,25(OH)2D3 signaling. Using either the MEK inhibitor UO126 or a non-phosphorylable RXR mutant, we can restore the cells nuclear import of RXR, VDR/RXR, as well as interaction with DNA and 1,25(OH)2D3 and VDR signaling.