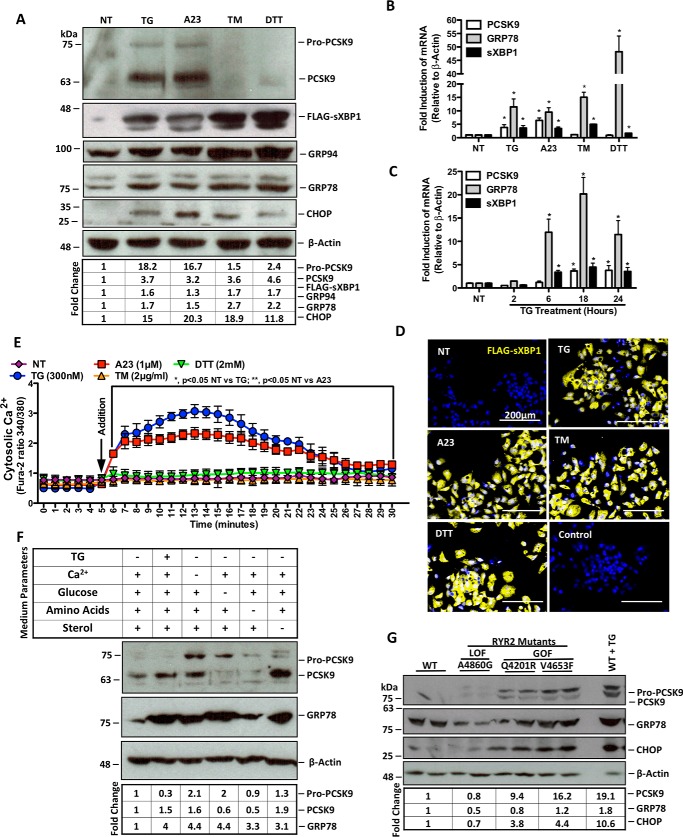
FIGURE 1.
ER Ca2+ depletion induces de novo PCSK9 expression. HuH7 cells were seeded in DMEM and transfected with the FLAG-sXBP1 ER stress reporter plasmid. Cells were subsequently untreated (NT) or treated with ER stress-inducing agents TG (100 nm), A23 (1 μm), TM (2 μg/ml), or DTT (2 mm) for 24 h. A and B, following treatment, protein lysates were collected for immunoblot analysis of PCSK9 and UPR markers FLAG-sXBP1, GRP94, GRP78, and CHOP. RNA was collected for qRT-PCR analysis of PCSK9, sXBP1, and GRP78. C, RNA from HuH7 cells treated with TG (100 nm) for 2, 6, 18, or 24 h were also examined via qRT-PCR to determine the time dependence of TG-induced PCSK9 expression. *, p < 0.05. D, HuH7 cells, which underwent the same transfection and treatment conditions as in A and B, were also stained for FLAG to examine UPR induction via immunofluorescence microscopy. E, HuH7 cells were plated in clear bottom 96-well plates and incubated with Fura-2AM, a fluorescent cytosolic Ca2+ indicator, for 30 min. Following incubation, cytosolic Ca2+ of cells treated with ER stress-inducing agents was measured for 30 min. F, to determine the effect of ER stress-inducing conditions via non-pharmacologic means on PCSK9 expression, HuH7 cells were exposed to medium deficient in either Ca2+, glucose, amino acids, or sterol for 24 h. G, in addition, the role of ER Ca2+ loss on PCSK9 expression was examined using HEK293 cells that stably overexpress tetracycline-inducible GOF or LOF RYR2 mutants using immunoblots. GOF RYR2 mutant cell lines (Q4201R and V4653F) exhibit increased ER Ca2+ leakage, whereas LOF RYR2 mutant (A4860G) exhibits reduced ER Ca2+ leak compared to WT cells (6, 42). Differences between treatments were assessed with paired Student's t tests, and all values are represented as mean ± S.D.