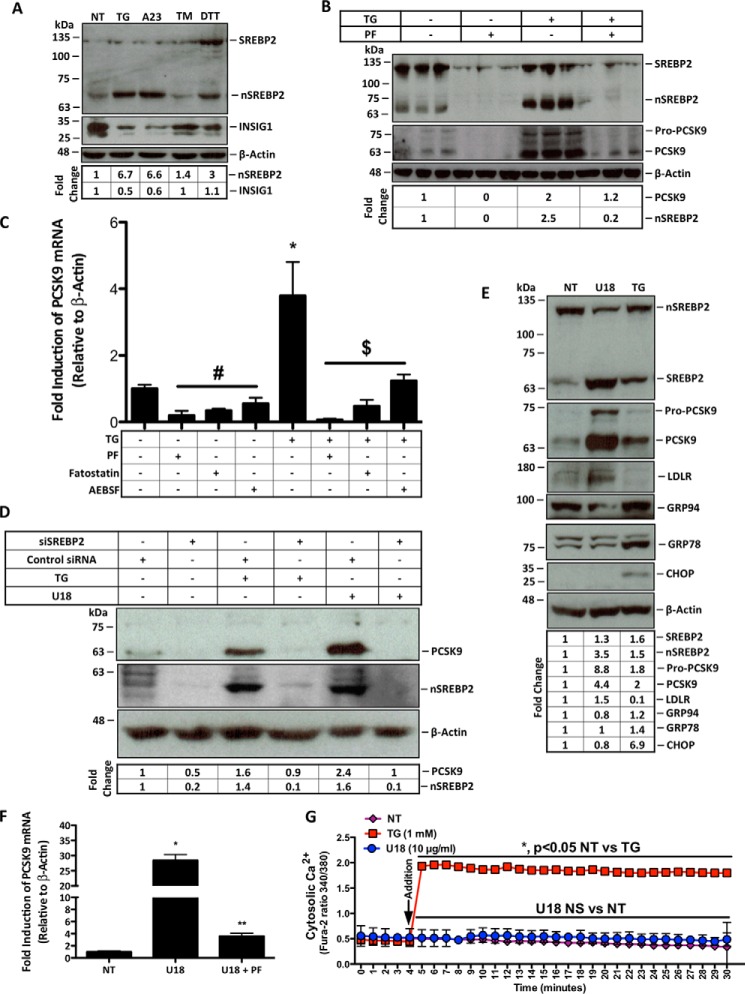
FIGURE 2.
ER Ca2+ depletion induces PCSK9 expression in an SREBP2-dependent manner. A, protein lysates were collected from HuH7 cells either untreated (NT) or treated with ER stress-inducing agents TG (100 nm), A23 (1 μm), TM (2 μg/ml), or DTT (2 mm) for 24 h for immunoblot analysis. B, HuH7 cells were then treated with TG in the presence or absence of PF (10 μm), which inhibits SREBP2 activation, for 24 h. Immunoblot analysis was carried out as in A to examine the SREBP2 dependence of PCSK9 expression resulting from ER Ca2+ release. C, RNA was collected from HuH7 cells, which were treated with additional inhibitors of SREBP2 activation, including AEBSF (0.3 mm) and fatostatin (20 μm) in the presence or absence of TG (100 nm) to further elucidate the SREBP2 dependence of PCSK9 expression. #, p < 0.05 versus untreated cells (NT); *, p < 0.05 versus NT; $, p < 0.05 versus TG-treated cells. D, to confirm the link between SREBP2 activation and PCSK9 expression in our model, HuH7 cells were transfected with SREBP2 siRNA. Following transfection, cells were also treated with TG (100 nm) or U18 (1 μg/ml), an inducer of SREBP2 activation, for 24 h. E, to examine SREBP2 activation by U18, immunoblots were used to directly assess SREBP2 and markers of SREBP2 activation, PCSK9 and LDLR; UPR markers GRP94, GRP78, and CHOP were also evaluated to determine the influence of U18 on UPR activation. F, PCSK9 mRNA was also examined in HuH7 cells treated with U18 in the presence or absence of PF for 24 h. *, p < 0.05 versus NT; **, p < 0.05 versus U18-treated cells. G, to assess whether U18-induced PCSK9 expression occurs in a Ca2+-dependent manner, HuH7 cells were incubated with fluorescent cytosolic Ca2+ indicator for 30 min and subsequently treated with U18 (10 μg/ml) or TG (1 mm) control. Differences between treatments were assessed with paired Student's t tests, and all values are represented as mean ± S.D.