Abstract
Purpose
To evaluate the efficacy of topical rapamycin in treating autoimmune dacryoadenitis in a mouse model of Sjögren's syndrome.
Methods
We developed rapamycin in a poly(ethylene glycol)-distearoyl phosphatidylethanolamine (PEG-DSPE) micelle formulation to maintain solubility. Rapamycin or PEG-DSPE eye drops (vehicle) were administered in a well-established Sjögren's syndrome disease model, the male nonobese diabetic (NOD) mice, twice daily for 12 weeks starting at 8 weeks of age. Mouse tear fluid was collected and tear Cathepsin S, a putative tear biomarker for Sjögren's syndrome, was measured. Lacrimal glands were retrieved for histological evaluation, and quantitative real-time PCR of genes associated with Sjögren's syndrome pathogenesis. Tear secretion was measured using phenol red threads, and corneal fluorescein staining was used to assess corneal integrity.
Results
Lymphocytic infiltration of lacrimal glands from rapamycin-treated mice was significantly (P = 0.0001) reduced by 3.8-fold relative to vehicle-treated mice after 12 weeks of treatment. Rapamycin, but not vehicle, treatment increased tear secretion and decreased corneal fluorescein staining after 12 weeks. In rapamycin-treated mice, Cathepsin S activity was significantly reduced by 3.75-fold in tears (P < 0.0001) and 1.68-fold in lacrimal gland lysates (P = 0.003) relative to vehicle-treated mice. Rapamycin significantly altered the expression of several genes linked to Sjögren's syndrome pathogenesis, including major histocompatibility complex II, TNF-α, IFN-γ, and IL-12a, as well as Akt3, an effector of autophagy.
Conclusions
Our findings suggest that topical rapamycin reduces autoimmune-mediated lacrimal gland inflammation while improving ocular surface integrity and tear secretion, and thus has potential for treating Sjögren's syndrome–associated dry eye.
Keywords: Sjögren's syndrome, rapamycin, autoimmune dacryoadenitis, Cathepsin S, nonobese diabetic mouse, dry eye, eye drops
Dry eye is a common ophthalmic pathology caused by either deficiencies in the production of the aqueous tear film or by imbalances in tear lipids that promote increased evaporation.1 Aqueous-deficient dry eye originates with decreased production and secretion of tears from the lacrimal gland (LG). This gland is the primary source of aqueous tears containing diverse proteins that protect and sustain the ocular surface.2 For aqueous-deficient dry eye, some of the most severe cases are associated with Sjögren's syndrome (SS), a chronic and systemic autoimmune disease that is characterized by lymphocytic infiltration and inflammation of the LG and salivary glands.1,3 Dry eye often presents as a gritty, itching, and burning sensation on the ocular surface, and in its severe form, including SS-associated dry eye, can lead to compromised visual function.4,5 The relationship between the decreased production of aqueous tears in SS-associated dry eye and the autoimmune inflammation is not yet clearly understood, as changes in the secretory machinery can occur before the presence of infiltrates as shown in the male nonobese diabetic (NOD) mouse model of SS.6 However, treatments reducing LG inflammation are linked to increased tear secretion.7,8 There are currently limited options for management of severe aqueous-deficient dry eye, including that due to SS.9 Most current therapies have their primary goal as alleviation of symptoms, without addressing the underlying cause of the aqueous tear deficiency.10 Restasis, an ophthalmic emulsion of 0.05% cyclosporine A (CsA), is one of the two prescription eye drops approved by the Food and Drug Administration (FDA) for the treatment of chronic dry eye caused by inflammation.11,12 Although it is commonly used in SS patients, most published clinical studies have been conducted in mixed SS and non-SS dry eye populations, making its actual efficacy in SS patients unclear.13–15 Studies indicate that only limited success has been achieved in the treatment of SS-associated dry eye,16 with the frequency of administration and the burning and itching sensation linked with its use17 associated with poor patient compliance. Efficacy of the recently FDA-approved dry eye drops Xiidra (5% Lifitegrast ophthalmic solution) for specific treatment of SS-associated dry eye has not yet been extensively evaluated.18–20 Other treatment options for dry eye include artificial tears for symptomatic relief and topical steroids for reduction of ocular surface inflammation. Steroids are associated with unwanted side effects, such as glaucoma and cataracts, thereby limiting their long-term use.21
The pathogenesis of SS remains unknown, although new advances have been enabled partly through studies in numerous mouse models of the disease. One well-established model is the NOD mouse, which spontaneously develops characteristics that resemble human SS, including autoimmune dacryoadenitis and inflammation of the salivary gland (sialoadenitis).22,23 The pattern of disease development and progression differs between males and females, with male NOD mice developing autoimmune dacryoadenitis but not sialoadenitis by 12 weeks of age.24 In contrast, female NOD mice first develop autoimmune sialoadenitis at approximately 12 to 20 weeks,25–27 but exhibit only mild LG inflammation at approximately 20 weeks.28 To avoid the confounding effects of diabetes and to maintain our focus on autoimmune dacryoadenitis, we have used the male NOD mouse as a model for SS-associated dry eye. In addition to developing lymphocytic infiltration in the LG, our work in this model has revealed upregulation of several mediators of inflammation in the diseased LG, including cytokines, such as IL-10, IL-12a, and IFN-γ, and lysosomal cysteine proteases, like Cathepsin S (CTSS) and Cathepsin H.29 Furthermore, we have shown increased activity of CTSS not only in the LG of male NOD mice but also in their tears. We recently confirmed this finding of elevated tear CTSS in SS patients,30 suggesting CTSS as a tear biomarker of SS-associated dry eye.
We have also recently demonstrated that autoimmune dacryoadenitis in male NOD mice is reduced with short-term intravenous treatment with a potent immunomodulatory agent, rapamycin (Rapa), also known as Sirolimus.31 Rapa is a macrolide antibiotic with potent immunosuppressive properties that acts by inhibiting the mammalian target of rapamycin (mTOR), a serine/threonine protein kinase that modulates several cellular functions, including protein and lipid synthesis and cell proliferation, including the proliferation of activated lymphocytes.32 Additionally, Rapa induces autophagy, a cellular process involving the targeting of damaged or aged macromolecules and organelles for lysosomal degradation. Rapa is regularly used as an immunosuppressant in solid organ transplantation rejection, and its use has also been recently exploited for certain autoimmune diseases and cancers.33–35 However, Rapa's systemic use has been limited by its side effects, including stomatitis,36 nephrotoxicity,37,38 and pulmonary toxicity.39 In our previous study, nephrotoxicity was observed even with short-term use (three doses over 1 week). However, when formulated with a Rapa-binding protein-polymer nanoparticle,40 Rapa's systemic side effects were greatly reduced.
Although effective in reducing dacryoadenitis in NOD mice, the intravenous delivery used in this previous study is not optimal for SS patients, especially if frequent injections are required to achieve therapeutic effects. Eye drops, the most popular dosage form for ocular diseases in clinical practice, are a natural and intuitive way for delivering medications to the ocular surface, especially if multiple administrations are required. Although the route is unknown, a number of studies have indicated that a limited dose of pharmaceuticals given by eye drops may reach the LG and can affect its functional status,8,41 making this approach especially valuable for SS-associated dry eye. However, a major drawback of Rapa is that it is highly hydrophobic and has very low water solubility, resulting in the need for excipients like ethanol, polysorbate 80, and polyethylene glycol 400 (PEG),42 some of which have detrimental effects on the ocular surface43,44 and that reduce patient compliance due to pain and irritation. Therefore, better formulations and techniques to enhance Rapa's aqueous solubility and bioavailability for eye drop administration, while reducing its unwanted side effects, would be highly desirable in pursuing the utility of Rapa eye drops.
The current study describes the development and optimization of an eye drop formulation of Rapa in poly(ethylene glycol)–distearoyl phosphatidylethanolamine (PEG-DSPE) in male NOD mice to determine its therapeutic efficacy in the treatment of autoimmune dacryoadenitis. We found that Rapa eye drops showed significant suppression of LG inflammation after 12 weeks of twice-daily treatment, while also improving tear secretion and reducing corneal fluorescein staining. In addition, Rapa also caused changes in diverse indicators of disease, decreasing tear CTSS activity and altering expression of LG genes putatively involved in the pathogenesis of SS, including the proinflammatory cytokines IL-12a, TNF-α, IFN-γ, major histocompatibility complex (MHC) II, and the autophagy regulator, Akt3. These findings are the first to demonstrate the ability of Rapa eye drops to treat autoimmune dacryoadenitis while improving tear secretion, suggesting this agent as a viable choice for treatment of SS-associated dry eye.
Materials and Methods
Mice
Male NOD mice at 8 weeks of age were used for the study, and were bred in-house from breeding pairs obtained from Taconic (Hudson, NY, USA). All animal use was in compliance with policies approved by the University of Southern California Institutional Animal Care and Use Committee, and experiments were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Reagents
Methoxylated poly(ethylene glycol)–distearoyl phosphatidylethanolamine (PEG-DSPE) was from Avanti Polar Lipids (Alabaster, AL, USA). Rapamycin was from LC Laboratories (Woburn, MA, USA). Restasis was from Allergan (Parsippany, NJ, USA). The reverse transcription kit, TaqMan Universal PCR Master Mix, and primers for real-time (RT)-PCR analysis of GAPDH (glyceraldehyde 3-phosphate dehydrogenase), ulk1, ulk2, Akt3, TNF-α, IFN-γ, MHCII, and IL-12a were from Life Technologies (Grand Island, NY, USA). The RNeasy plus Universal Mini Kit and the RNeasy Plus Micro Kit were from Qiagen (Valencia, CA, USA). The CTSS activity assay kit was from Biovision (Milpitas, CA, USA). Microcaps microcapillary tubes were from Drummond (Broomall, PA, USA). ZoneQuick phenol red threads were purchased from Oasis Medical (Glendale, CA, USA). Fluorescein sodium strips (FUL-GLO) were from Akorn, Inc. (Buffalo Grove, IL, USA). PEN membrane-coated slides for LG sections used for laser capture microdissection were bought from ThermoFisher Scientific (Canoga Park, CA, USA). The eye relief solution used to wash the mouse ocular surface was purchased from Bausch & Lomb (Rochester, NY, USA). Ketamine (Ketaject) was from Phoenix (St. Joseph, MO, USA) and xylazine (AnaSed) was from Akorn (Lake Forest, IL, USA). Carbamylcholine, used as a secretagogue to stimulate tear production, was from Sigma-Aldrich (St. Louis, MO, USA). Free Style Lite test strips were from Abbott Diabetes Care, Inc. (Alameda, CA, USA). The Bio-Rad protein assay kit was from Bio-Rad (Hercules, CA, USA), and 0.2-μm cellulose acetate syringe filters were from VWR (Radnor, PA, USA).
Size Determination of PEG-DSPE
The hydrodynamic radius of PEG-DSPE micelles was determined using dynamic light scattering, as previously described.45,46 Samples were prepared at 25 μM in PBS and passed through Anotop 10 filters with 0.02-μm pores (GE Healthcare Bio-Sciences, Pittsburg, PA, USA); 80 μL of the sample was pipetted into a 384-well clear bottom microplate (Greiner-Bio, Monroe, NC, USA) in triplicate followed by addition of 20 μL mineral oil to prevent evaporation. The plate was then centrifuged at 25°C at 1700g for 10 minutes to remove air bubbles. Dynamic light-scattering data were collected at 25°C, with an 830-nm wavelength laser at an angle of 158°C using a DynaPro Plate Reader II (Wyatt Technology, Santa Barbara, CA, USA). Data were analyzed using the DYNAMICS software supplied by the manufacturer, which uses the Stokes-Einstein equation to estimate the hydrodynamic radius.
Encapsulation of Rapa in PEG-DSPE and Solubility Determination
Rapa was solvated in PEG-DSPE, which forms micelle nanoparticles. The method for solvation consisted of combining the lipid polymer in chloroform with Rapa in methanol in a 1:1 ratio and evaporating the mixture in a rotary evaporator, leading to the formation of a thin film, which was then re-solubilized in PBS. Any insoluble Rapa was removed from the micelle/drug mixture by centrifugation at 2350g for 10 minutes followed by filtration of the resulting solution through a 0.2-μm cellulose acetate syringe filter. The vehicle (empty micelle) was prepared using the same method in the absence of drug. The total amount of encapsulated material in the micelle was then measured using a reverse-phase high-performance liquid chromatography (RP-HPLC) method consisting of H2O and methanol in a gradient ranging from 40% to 100%. The final concentration was determined by using a standard curve with the encapsulation efficiency of PEG-DSPE being approximately 60% to 70%. To estimate the water solubility of Rapa in PEG-DSPE, encapsulation was followed by lyophilization in a preweighed glass vial. The dried sample was reweighed and resuspended in water assisted by sonication. Solubility was estimated by measuring the volume of water required to completely dissolve the drug after adjusting for the weight of the PEG-DSPE.
Rapa Release From PEG-DSPE Micelles
Rapa release from the nanoparticle formulation was done by dialysis under sink conditions in PBS at room temperature. A 3.5-kDa cutoff dialysis cassette (Life Technologies) was loaded with PEG-DSPE-Rapa, and samples were collected from the cassette at intervals from 0 to 48 hours. To ensure that Rapa's diffusion across the dialysis membrane was not the rate-limiting step, a small amount of free Rapa in 3% dimethyl sulfoxide was dispersed in a dialysis cassette and samples were collected at appropriate time intervals. The amount of Rapa retained was determined by using RP-HPLC, as described above. Nonlinear regression was used to calculate the release half-life of Rapa from PEG-DSPE micelles.
Determination of Rapa in Plasma, LG, and Draining Lymph Nodes of Male NOD Mice
To explore whether or not any drug reached the plasma, LG, or draining lymph nodes after eye drop administration, liquid chromatography-mass spectrometry (LC-MS) analysis was used to determine the concentration in tissue and plasma samples. Rapa was administered either as eye drops or intravenously as a positive control, whereas eye drop vehicle was used as a negative control (n = 3). Lacrimal gland, draining lymph nodes, and blood of the treated mice were collected 2 hours after administration. Blood was centrifuged at 400g at 4°C for 10 minutes and plasma was separated and frozen until LC-MS analysis, performed as described previously.31 For mouse plasma samples, 25 μL of 500 ng/mL tacrolimus (Fujisawa, Deerfield, IL, USA) was added as the internal standard to a 50-μL aliquot of plasma sample followed by addition of 350 μL acetonitrile to each sample to extract Rapa and internal standard. For lymph nodes and LG, samples were weighed, and a bullet blender (Next Advance, Inc., Averill Park, NY, USA) was used to homogenize the tissues; 25 μL internal standard was added to each sample and cold acetonitrile was used to precipitate protein. Samples were then incubated at −20°C for 1 hour and the entire mixture was vortexed and centrifuged at 13,000g for 5 minutes; 40 μL supernatant was then transferred to HPLC vials, and 30 μL was injected into the LC-MS for detection. Rapa and tacrolimus were separated using a Kinetex EVO C18 column (Phenomenex, Torrance, CA, USA) with the following dimensions: 50 × 3.0 mm × 5 μm (P/No. 00B-4433-Y0; S/No. 733389–5). A Shimadzu (Columbia, MD, USA) LC-20AD HPLC linked to an API4000 mass spectrometer equipped with an electrospray ionization mode was used to quantify Rapa and tacrolimus via multiple-reaction-monitoring of 931.6 → 864.8 and 821.6 → 768.4, respectively. Mobile phase A was water with 0.5% formic acid, whereas mobile phase B was acetonitrile with 0.5% formic acid. Samples were eluted using a solvent gradient of mobile phase B from 50% to 90% over 1 minute and held constant for another 2 minutes.
Eye Drop Studies in Male NOD Mice
For Rapa eye drop treatments, we used male NOD mice, aged 8 weeks at the onset of treatment. At this age, the LGs in these mice are at early stages of inflammation.29 Mice were treated with 2 μL of 550 μM (0.05%) encapsulated PEG-DSPE-Rapa for 12 weeks with PEG-DSPE (vehicle) serving as control. The study was performed in two additional cohorts of mice, using alternative controls; in one, Rapa eye drop treatment was compared with PBS and in the other, Rapa eye drop treatment was compared with Restasis (0.05%), PEG-DSPE alone (vehicle), and PBS. At the end of the study, mice were intraperitoneally administered a mix of 50 to 60 mg ketamine and 5 to 10 mg xylazine per kilogram of body weight and euthanized by cervical dislocation immediately after stimulated tear fluid collection described below. The extraorbital LGs were isolated and either fixed in 10% neutral-buffered formalin, or placed in lysis buffer and homogenized to extract total RNA for quantitative (real-time) PCR (qPCR) analysis or to prepare lysates for CTSS activity assay. To control for the development of diabetes in the study cohort, blood glucose levels in peripheral blood obtained by a tail nick were monitored using Free Style Lite (Abbott Diabetes Care, Inc., Alameda, CA, USA) test strips, before the start of the treatment (8 weeks of age), after 6 weeks of treatment (14 weeks of age), and after the completion of treatments (20 weeks of age).
Stimulated Tear Collection
For collection of tear fluid, a small incision was made bilaterally on an axis between the outer junction of the eyelid and the ear of the mice, thus exposing the LGs. The transparent connective tissue capsule wrapped around the LG was carefully removed and a small piece of cellulose mesh (Kimwipe; Fisher Scientific, Pittsburgh, PA, USA) was placed on the gland to hold the stimulant (3 μL of 50 μM carbachol [CCH]). The eyes were washed with eye relief solution before adding CCH. After stimulating the gland, tears from both eyes were carefully collected by using 2 μL microcapillary tubes by gently placing it at the tear meniscus in the medial canthus for 5 minutes. Stimulation was performed three times for a total collection time of 15 minutes and the volume of collected tears was recorded and then carefully emptied into sterile tubes and placed on ice until further analysis.
Basal Tear Production
Before measurement of aqueous tear production, the mice were anesthetized briefly using continuous flow of isoflurane through a nose cone. While under light anesthesia, a ZoneQuick (Oasis Medical) phenol red thread was carefully inserted under the lower eyelid for 10 seconds and tear production was reported as a function of the length of wetting of the thread in millimeters, which was measured using a loupe with a millimeter scale. This was repeated for both eyes. The data presented are an average of wetting length of the threads from both eyes.
Corneal Fluorescein Staining
Corneal staining was performed before onset of treatment and after 6 and 12 weeks of treatment. Strips containing 0.6 mg fluorescein sodium (FUL-GLO) were incubated in 200 μL PBS to serve as contrast solution, of which 0.75 μL was applied to the cornea of mice anesthetized using isoflurane. The eye was blinked five times, and allowed to stand for 1 minute before photographs were obtained using a Canon EOS 5D Mark II (Melville, NY, USA) attached to a stereoscopic microscope (Motic, Richmond, BC, Canada) during ocular illumination with a cobalt blue light. Corneal staining was graded by a blinded reviewer using the system described in a National Eye Institute Workshop47 in which each of the five areas of the cornea are scored from 0 to 3 and summed. The scores from the left and right eyes were averaged.
Analysis of LG Inflammation, Conjunctival Inflammation, and Conjunctival Goblet Cell Density
Lacrimal gland histology and quantification of inflammation was performed as described previously.31 Briefly, paraffin sections of LG were stained with hematoxylin-eosin (H&E) according to standard procedures and photographed using a Nikon 80i microscope (Melville, NY, USA) equipped with a digital camera. Images of three nonconsecutive whole gland cross sections per gland were obtained for each LG. The area of the LG occupied by lymphocytic infiltrates was calculated by using ImageJ software (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA) by a blinded reviewer. In paraffin-embedded sections of bulbar and palpebral conjunctiva stained with periodic acid-Schiff's stain and Alcian Blue in three nonconsecutive slides with five serial sections on each from 13 mice in the drug group and 14 mice in the vehicle group, a blinded, trained pathologist counted stained goblet cells as described previously.48 The conjunctival sections were also examined by the same blinded reviewer for the presence of inflammatory cells and a new grading system was developed as follows: 0, no evidence of chronic inflammation (no lymphocytes identified underneath the conjunctival epithelium; note: rare intraepithelial lymphocytes may be present and are a part of normal conjunctival associated lymphoid tissue); 1, very mild chronic inflammation (a few lymphocytes identified underneath the conjunctival epithelium); 2, mild interspersed chronic inflammation (many scattered lymphocytes identified underneath the conjunctival epithelium); 3, focus of chronic inflammation (distinct aggregate/follicle of lymphocytes identified underneath the conjunctival epithelium); 4, severe chronic inflammation (numerous lymphocytes and distinct aggregates/follicles of lymphocytes identified underneath the conjunctival epithelium).
Cathepsin S Activity Analysis
Cathepsin S activity in tears and LG lysates of Rapa-treated mice was compared to levels in tears and LG lysates of vehicle-treated mice by using a protocol modified from that described previously.29,31 Briefly, for tear CTSS analysis, stimulated tear fluid was diluted to 200 μL of CTSS reaction buffer and divided into two 100-μL reactions on a 96-well plate. For CTSS analysis in lysates, 10 μL clarified LG lysate plus 40 μL lysis buffer plus 50 μL reaction buffer was added to each of two wells. Cathepsin S–specific inhibitor was added to one of the two wells for each sample. After the experiment, the Bio-Rad protein assay was performed and the activity was normalized to 50 μg total protein.
Real-Time PCR
For measurement of gene expression, total RNA was isolated from whole LG and cornea (three corneas originating from three different mice were pooled) of Rapa- and vehicle-treated mice by using the RNeasy plus Universal Mini Kit per the manufacturer's protocol. To determine CTSS expression levels in LG acinar cells versus infiltrating immune cells within the LG, LGs were collected in RNase-free conditions, placed in optimal cutting temperature (OCT) compound and flash frozen in liquid nitrogen (n = 3 per treatment). These glands were sectioned immediately on membrane-coated slides and stained with hematoxylin. A laser microdissection microscope (Leica Microsystems, Deerfield, IL, USA) was used to collect acinar cells and infiltrating lymphocytes from these sections under RNase-free conditions as described previously49 and total RNA was isolated using the RNeasy Plus Micro Kit. Complementary DNA was prepared by using the reverse transcription kit as per the manufacturer's recommendations. Quantitative (real-time) PCR was performed with the TaqMan gene expression assays on the ABI 7900HT real-time PCR system, using the following probes: TNF-α (Mm00443258_m1), IFN-γ (Mm01168134_m1), ulk1 (Mm00437238_m1), ulk2 (Mm03048846_m1), Akt3 (Mm00442194_m1), MHCII (Mm00439216_m1), and IL-12a (Mm00434165_m1); GAPDH (Mm99999915_g1) was used as a control housekeeping gene. Each reaction consisted of 1 μL cDNA from the reverse transcription reaction mixed with 8 μL nuclease-free water and 1 μL assay primer mixed with 10 μL TaqMan Universal PCR Master Mix. Each sample was run in triplicate. The thermal profile consisted of preheating the samples at 95°C for 10 minutes followed by 40 repeats at 95°C for 15 seconds each and 60°C for 1 minute. The relative expression levels were calculated using the comparative CT method (ΔΔCT method) on the default ABI software SDS 2.3 (ThermoFisher Scientific, Waltham, MA, USA) as described previously.50
Statistics
All statistical analyses were performed using GraphPad Prism software (GraphPad, San Diego, CA, USA). Shapiro Wilk, KS, and D'Agostino & Pearson omnibus normality tests were used to test for normal distribution in each data set. For normally distributed data, a two-tailed, unpaired Student's t-test was used to compare differences between two treatment groups, whereas the Mann-Whitney test was used for nonparametric analysis. Three treatment groups or more were compared using 1-way ANOVA with the Tukey's HSD test for post hoc analysis within those groups. A P ≤ 0.05 was considered as a significant difference.
Results
Characterization of Rapa Eye Drop Formulation and Bioavailability
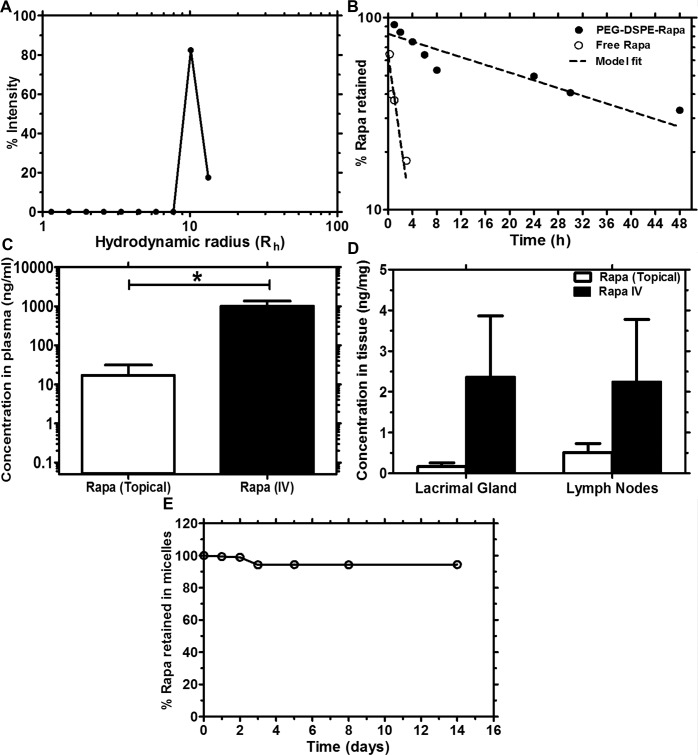
The vehicle used to encapsulate Rapa, PEG-DSPE, is known to form nano-sized micelles with a low critical micelle concentration.51,52 We confirmed the size of these nanoparticles using dynamic light scattering, which has a hydrodynamic radius of approximately 11 nm (Fig. 1A). The in vitro drug release of Rapa from PEG-DSPE micelles was evaluated in PBS under sink conditions, with our results showing a slow rate of release with 50% of the drug retained over approximately 29.9 hours (Fig. 1B). In contrast, 50% of the free drug was released within 1.35 hours, indicating that the release rate of Rapa from PEG-DSPE micelles was indeed the rate-limiting step during this in vitro release assay. The solubility of Rapa in PEG-DSPE was found to be 2.6 mg/mL. This simple micelle formulation increased the solubility of rapamycin by 1000-fold as compared with the reported aqueous solubility of rapamycin (2.6 μg/mL). It has been reported that a limited amount of another drug, timolol, can reach the systemic circulation via absorption in the nasolacrimal duct when delivered topically on the ocular surface.53,54 To explore the possibility that Rapa administered in eye drops could reach the LG through the systemic circulation or the lymphatic system, we collected blood, LG, and draining lymph nodes from mice that were treated with Rapa via either eye drops (40 μg/kg or 0.05% dose) or intravenous injection (0.75 mg/kg dose) 2 hours after administration. These doses were selected for evaluation because they both reduce indicators of dacryoadenitis in the male NOD mice.31 Plasma/tissue Rapa concentration was analyzed with LC-MS. Rapa was detectable in the plasma of mice that were treated with Rapa eye drops, although the concentration was lower in mice receiving Rapa through eye drops (Fig. 1C). Rapa was similarly detected in small amounts in the LG and draining lymph nodes of not only those mice that were treated with intravenous Rapa but also the mice that received Rapa eye drops (Fig. 1D). At the selected dosages, the drug concentrations detected in the plasma, LG, and lymph node were lower for the topical administration than for intravenous administration. However, when the ratio of drug in tissue to plasma was calculated for both routes of administration, there was a 4-fold increase in LG/plasma ratio and a 14-fold increase in the LN/plasma ratio in the topical group relative to the intravenous group. This suggests topical eye drops shift the biodistribution of drug toward the target tissue, which may be advantageous in reducing toxicity prevalent in systemic administration. The storage stability of Rapa-loaded micelles was also determined for a period of 14 days. As shown in Figure 1E, there was approximately 95% retention of Rapa in the micelles over this period, suggesting good storage stability of the drug-loaded micelles at −20°C. For Rapa treatment over 12 weeks, fresh eye drop aliquots were prepared weekly to ensure stability.
Figure 1.
Detection of Rapa in systemic circulation after eye drop administration in a nanoparticle lipid polymer eye drop formulation. (A) Dynamic light-scattering analysis showed that PEG-DSPE formed nanoparticles with a hydrodynamic radius of approximately 11 nm. (B) Drug release from PEG-DSPE, as determined by dialysis in PBS at room temperature, demonstrated that drug release follows a one-phase decay model with a half-life of 29.9 hours, whereas the free drug diffuses rapidly through the dialysis membrane with a half-life of 1.35 hours. (C, D) To determine the serum and tissue availability of Rapa administered as eye drops, peripheral blood, LG, and draining lymph nodes were collected 2 hours after administration of vehicle or Rapa as eye drops or intravenously, and LC-MS analysis was used to determine the plasma concentration. n = 3 for each group. Error bars represent mean ± SD. Rapa could not be detected in the vehicle group (not shown) because it was below the limit of detection (0.05 ng/mL). (E) To determine the stability of PEG-DSPE-Rapa frozen aliquots (−20°C), the amount of drug retained in micelles was determined over 14 days. Reverse-phase HPLC analysis showed that the micelles retained approximately 95% of the drug over that time period.
Effect of Topical Rapa on Autoimmune Dacryoadenitis in NOD Mice
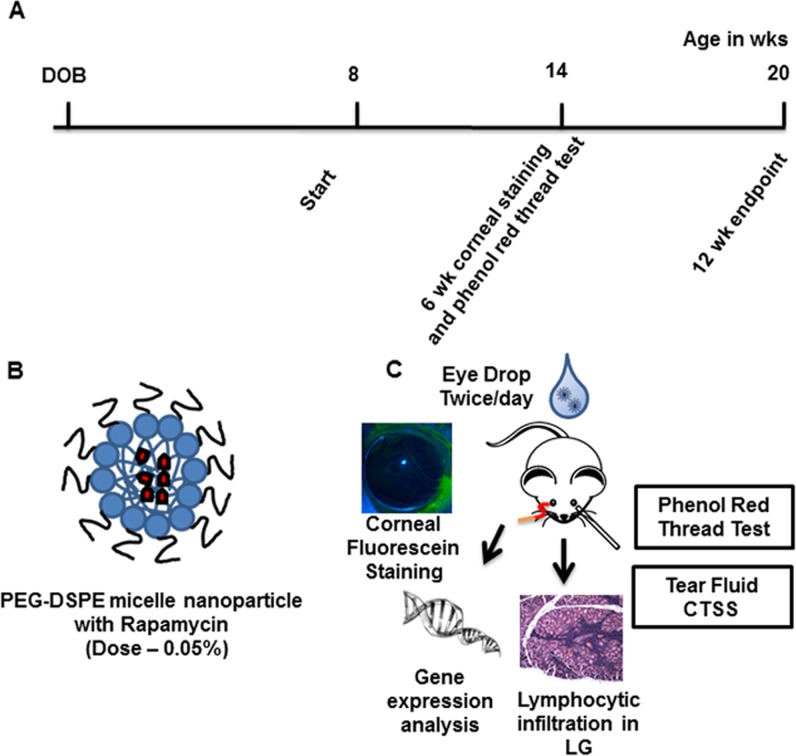
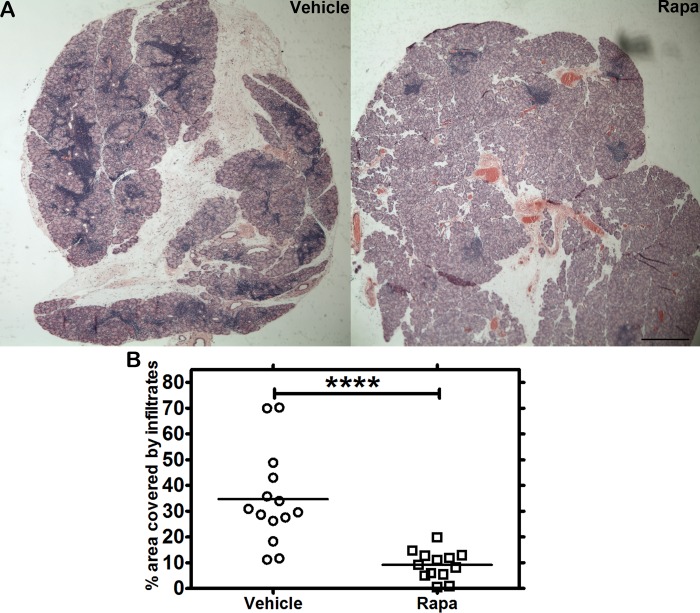
Male NOD mice exhibit lymphocytic infiltration in the LG in conjunction with LG dysfunction and reduced tear flow.55,56 In H&E-stained LG sections, these infiltrates resemble dark purple patches, which represent the nuclei of the lymphocytic foci. Previously, we showed that intravenous Rapa administered over 1 week to 12-week old male NOD mice significantly reduced lymphocytic infiltration in the LG.31 To evaluate the potential effect on the extent of lymphocytic infiltration by Rapa administered through eye drops, this report explores twice-daily instillation into eyes of male NOD mice for 12 weeks at a dose of 550 μM Rapa (0.05 % by mass), starting at 8 weeks of age when LG inflammation in male NOD mice is initiated but not fully developed (Fig. 2). The duration of this study is consistent with other studies evaluating the effect of CsA on LG inflammation and tear secretion in several different mouse models of SS.8 As seen in Figure 3, both H&E-labeled sections and image analysis quantifying the extent of lymphocytic infiltration showed a statistically significant reduction in Rapa-treated mice after 12 weeks of treatment when mice were 20 weeks of age, as compared with those treated with vehicle. Because Rapa was detected in plasma (Fig. 1C), histological evaluation of liver, lung, and kidney was also performed in five mice in each of the vehicle and drug treatment groups to evaluate signs of systemic toxicity. A trained pathologist conducted a blinded review of all the tissue sections. Qualitative histopathological differences were found in the lungs of two of five vehicle and one of five Rapa-treated mice, and in the liver of one of five vehicle-treated mice. No changes were observed in the kidneys of any of the analyzed mice from either group. The changes observed in the lungs and livers of these mice were nonspecific and in an appropriate setting, best fit with changes seen in early SS57–59 (Supplemental Table S1).
Figure 2.
Experimental design for investigation of the effects of Rapa eye drops on autoimmune dacryoadenitis. (A) Timeline depicting treatment of NOD mice starting in the early stages of autoimmune dacryoadenitis at 8 weeks of age. Treatments (0.05% Rapa by mass) were given over 12 weeks. (B) Cartoon depicting Rapa solubilized/encapsulated in PEG-DSPE micelle nanoparticles. (C) Diagram showing several therapeutic end points investigated after Rapa eye drop administration, which determined the treatment efficacy on autoimmune dacryoadenitis, tear flow, and ocular surface integrity.
Figure 3.
Hematoxylin-eosin staining of LG from NOD mice after Rapa eye drops reveals suppression of lymphocytic infiltration. (A) Male NOD mice were treated with 0.05% Rapa (PEG-DSPE micelles) or vehicle (PEG-DSPE alone) eye drops two times daily for 12 weeks starting at 8 weeks of age. Lacrimal gland sections from treated mice were stained with H&E, and representative sections are shown. (B) Lymphocytic infiltration was quantified as described in the Methods section, showing a significant decrease in infiltration in LG from mice treated with Rapa for 12 weeks relative to its vehicle. Image analysis of the LG shows percentage of area with lymphocytic infiltrates relative to the entire LG section. ****P < 0.0001. Crossbar represents the mean. Scale bar: 300 μm.
Because some male NOD mice develop diabetes at approximately 5 to 6 months, which could complicate interpretation of results, blood glucose levels were measured before treatments at 8 weeks of age, after 6 weeks of treatment at 14 weeks of age, and after 12-week treatment at 20 weeks of age. None of the enrolled mice had blood glucose levels higher than 150 mg/dL at the end of treatments when they had reached 20 weeks of age. This is consistent with previous reports of a 10% to 30% incidence of diabetes at 30 weeks of age in male NOD mice where the onset is seen at 20 weeks of age.60,61 Mice with blood glucose levels >250 mg/dL are usually considered diabetic.62 No significant differences in blood glucose levels between Rapa- and vehicle-treated groups were detected.
In a separate study cohort, Rapa eye drop treatment was compared with PBS eye drops, with similar results (Supplemental Fig. S1A). Having observed a significant effect on NOD mice with the 12-week treatment of Rapa eye drops, we conducted the same study in a third cohort, comparing our formulation with Restasis. We found that Rapa but not Restasis significantly reduced LG inflammation relative to PBS and vehicle controls, although a nonsignificant trend to reduced inflammation was seen for Restasis (Supplemental Fig. S1B).
Rapa Treatment Improves Clinical Signs Associated With Autoimmune-Mediated Dry Eye
To assess the potential therapeutic effect of Rapa eye drops in male NOD mice, corneal fluorescein staining was performed after 6 and 12 weeks of treatment. Figure 4A shows representative images of fluorescein-stained corneas from both the vehicle and the drug treatment groups after 12 weeks of treatment. Although corneal fluorescein scores increased in both Rapa- and vehicle-treated mice from baseline to 6 weeks, Rapa eye drops significantly decreased corneal staining when the scores were measured after 12 weeks relative to the vehicle treatment group (Fig. 4B). In addition, the amount of reflex tears was increased in Rapa-treated mice after 6 weeks of treatment as compared with the pretreatment value as measured by the wetting of phenol red threads in millimeters (Fig. 5A). This improvement in tear secretion continued even after 12 weeks of Rapa treatment. However, tear secretion declined in vehicle-treated mice from pretreatment to 6-week treatment and was similarly low at the end of the 12-week treatment period. We also measured stimulated tear secretion after 12 weeks of treatment and observed a modest, but statistically significant increase in stimulated tear production from Rapa-treated mice as compared with vehicle-treated mice (Fig. 5B). Finally, a blinded, trained pathologist analyzed the conjunctivas of Rapa and vehicle eye drop–treated mice after 12 weeks of treatment to quantify any differences in the density of conjunctival goblet cells and to measure the level of inflammatory infiltrates; however, no significant difference in conjunctival morphology was detected. Using the scoring system developed by the pathologist, we found no significant difference in the level of inflammation between the vehicle- and Rapa-treated conjunctivas. Representative images are shown in Supplemental Figure S2A. The mean conjunctival infiltration score in the vehicle group was 0.59 ± 1.12, whereas that in the Rapa group was 0.54 ± 0.96. No differences in goblet cell density were also detected (Supplemental Fig. S2B).
Figure 4.
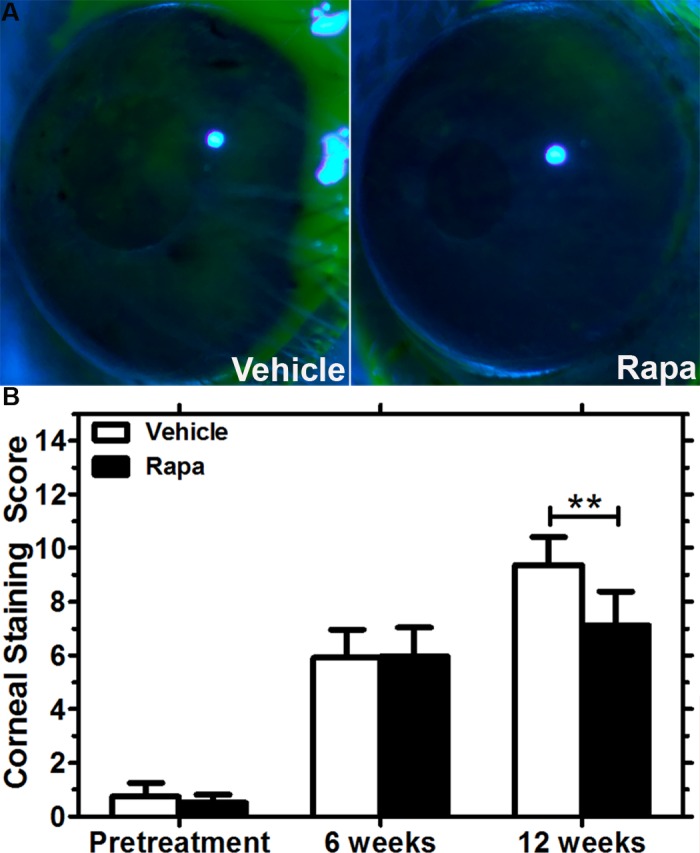
Improvement in ocular surface integrity in male NOD mice treated with Rapa eye drops for 12 weeks relative to the vehicle alone. (A) Representative images of the fluorescein-stained corneal surface of the vehicle- and Rapa-treated mice after 12 weeks of treatment. (B) Corneal fluorescein staining scores increased from levels at study onset (pretreatment) at 6 weeks and 12 weeks for both the vehicle- (n = 14) and Rapa-treated mice (n = 13); however, Rapa eye drops significantly lowered the score at 12 weeks relative to scores in vehicle-treated mice (**P < 0.01). Data are presented as mean ± 95% CI.
Figure 5.
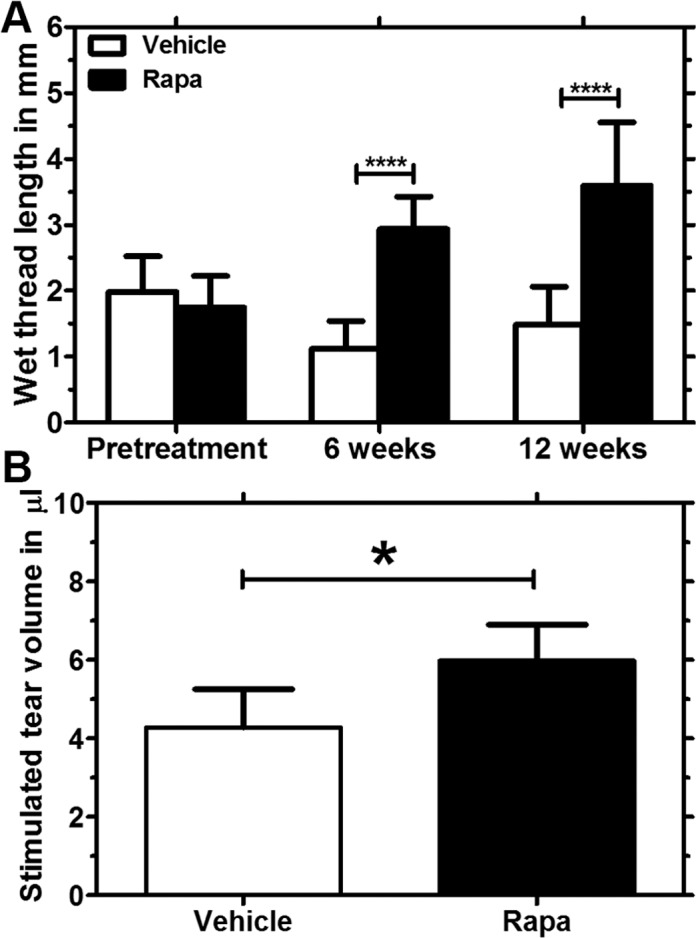
Tear secretion improved in Rapa-treated mice relative to the vehicle group. (A) A phenol red test was used to measure tear secretion and results are reported as wetting of phenol red threads in millimeters. When compared with the vehicle treatment, male NOD mice treated with Rapa (n = 13) showed a statistically significant increase in tear secretion after 6 weeks and the difference between the groups was even higher from 6 weeks to 12 weeks (****P < 0.0001). There was a significant decrease in tear secretion within the vehicle-treated group from pretreatment to 6 weeks, which appeared comparable after 12 weeks of treatment. (B) As a direct measurement of aqueous tear secretion after 12 weeks of Rapa treatment, we determined the volume of fluid secreted to the ocular surface on stimulation of the LG with CCH. The study was powered sufficiently to detect a modest, but significant increase in stimulated tear volumes for the drug (n = 9) compared with the vehicle (n = 10) treatment group (*P < 0.05). Data are presented as mean ± 95% CI.
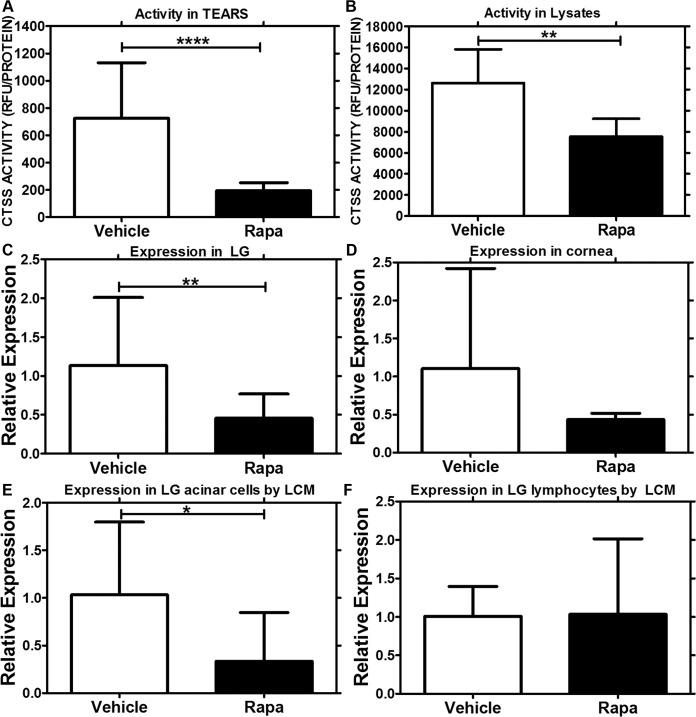
Topical Rapa Administration Reduces Tear and LG CTSS
Previous work from our laboratory has demonstrated that CTSS expression and activity in NOD mouse LG and tear fluid are significantly increased in autoimmune dacryoadenitis.29 Our recent clinical study has confirmed significant elevation of CTSS activity in tears from SS patients, suggesting that the increased tear fluid CTSS activity might serve as a biomarker for diagnosis of SS.30 Other findings in salivary gland suggest that CTSS upregulation may activate MHCII-mediated antigen presentation by salivary gland acinar cells, potentially triggering and perpetuating autoimmune responses.63 To examine the effect of long-term topical administration of Rapa eye drops on CTSS, we compared changes in its activity in tears and LG lysates from NOD mice without and with treatments. Our results indicated that treatment with Rapa eye drops reduced CTSS activity significantly in tears and LG lysates from NOD mice, as compared with those treated with vehicle (Figs. 6A, 6B). We further evaluated the changes in gene expression of CTSS in LG and cornea of these mice, observing a significant reduction in CTSS transcript levels in the LG of mice treated with Rapa relative to the vehicle group (Fig. 6C). A similar trend was observed in the cornea, although it was not statistically significant (Fig. 6D). Furthermore, as CTSS is expressed in both LG acinar cells and infiltrating lymphocytes in the diseased LG, to identify the cells showing the decreased CTSS expression in LG from Rapa-treated mice, we separately isolated RNA from LG acinar cells and infiltrating lymphocytes collected by laser capture microdissection and used qPCR to detect differential expression of CTSS in these cell types. Rapa treatment significantly decreased CTSS expression in LG acinar cells but did not affect CTSS expression in the remaining infiltrating lymphocytes (Figs. 6E, 6F).
Figure 6.
Decrease in CTSS activity and gene expression in tears, LG lysates, cornea, and LG acinar cells from mice treated with Rapa eye drops. (A) Tear CTSS activity measurements indicated a 6-fold decrease (P < 0.0001) in mice treated with Rapa eye drops (n = 9), as compared with those treated with vehicle alone (n = 10). (B) Cathepsin S activity measurements in LG lysates also demonstrated a significant decrease (P = 0.003) in mice treated with Rapa (n = 6), as compared with those treated with vehicle (n = 5). (C) Quantitative (real-time) PCR studies demonstrated that the CTSS mRNA level was significantly lower in whole LG (P = 0.008) in mice treated with Rapa (n = 5), as compared with those treated with vehicle alone (n = 5). (D) Although we saw a trend in CTSS reduction in cornea of mice treated with Rapa (n = 3), it was not statistically significant (P = 0.15). To determine if CTSS expression was modified in LG acinar cells or invading lymphocytes, we used laser capture microdissection to isolate individual acini and lymphocytes from hematoxylin-stained LG sections of Rapa- (n = 3) and vehicle- (n = 3) treated mice and carried out qPCR on these cell types. As can be seen in (E), there was a significant reduction in CTSS mRNA expression in the LG acinar cells with Rapa treatment when compared with the vehicle treatment group (P = 0.04); (F) CTSS mRNA expression was unchanged in the lymphocytes. Data are presented as mean ± 95% CI.
Effect of Rapa Eye Drops on LG Gene Expression
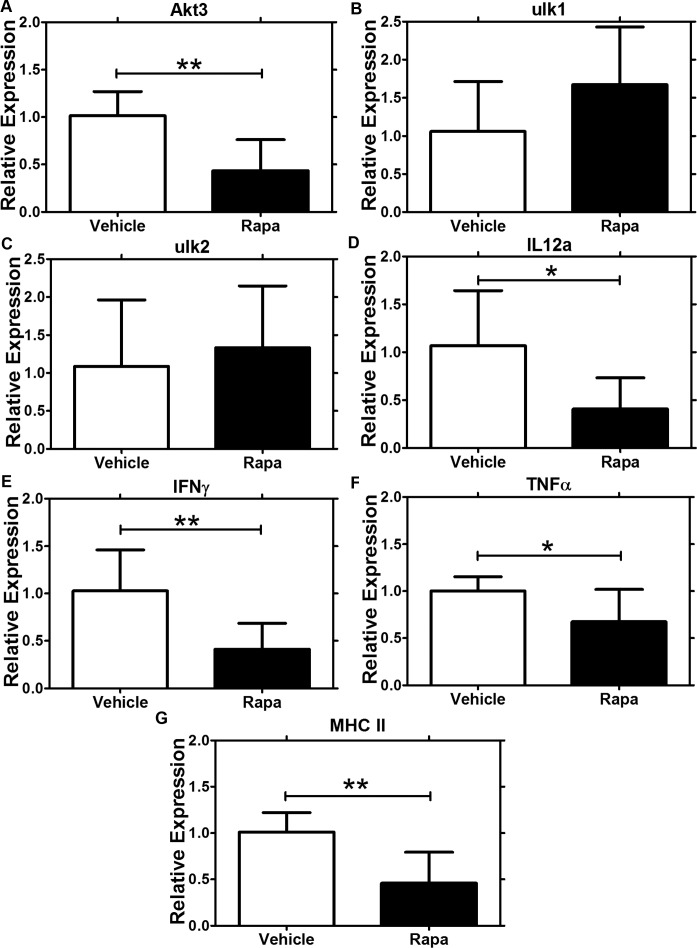
Systemic intravenous administration of Rapa over 1 week caused significant molecular changes associated with inflammatory and mTOR-related downstream signaling events in LG from NOD mice.31 In the present study, we analyzed the changes in genes that were previously shown to be affected by systemic Rapa and that may be targets of the therapeutic effect of Rapa in NOD mouse LG. We have hypothesized that induction of autophagy in the LG may help reduce LG inflammation by clearing accumulated proteins and damaged secretory vesicles, consistent with findings in salivary gland acini.64 Rapa caused a significant decrease in Akt3 gene expression levels (Fig. 7A), inhibition of which induces autophagy,65 but did not significantly alter expression of ulk1 (Fig. 7B) and ulk2 (Fig. 7C), positive effectors of autophagy.66 Gene expression of IFN-γ, TNF-α, and IL-12a (Figs. 7D–F), linked to LG inflammation in SS,29 and MHCII (Fig. 7G), involved in antigen presentation, were decreased in LG from mice treated with Rapa eye drops, compared with those treated with vehicle.
Figure 7.
Quantitative (real-time) PCR studies demonstrated changes in gene expression of proteins involved in autophagy, inflammation, and antigen presentation in NOD mice treated with Rapa eye drops. In LG from male NOD mice treated with Rapa eye drops or vehicle for 12 weeks, Rapa treatment caused downregulation of (A) Akt3 (**P = 0.005) but did not alter expression of (B) ulk1 or (C) ulk2 relative to levels seen in LG from mice treated with vehicle eye drops. Treatment with Rapa eye drops also caused a significant decrease in gene expression of (D) IL-12a (*P = 0.03), (E) IFN-γ (*P = 0.05), (F) TNF-α (**P = 0.007), and (G) MHCII (**P = 0.006). Data are presented as mean ± 95% CI; n = 5 for each group.
Discussion
At present, SS-associated dry eye is treated with artificial tears that lubricate the ocular surface, and/or with topical anti-inflammatory agents like CsA formulated in Restasis, which reduce ocular surface inflammation. Although reasonably effective in treating ocular surface symptoms, the exploration of topical administration of anti-inflammatory agents that reduce the autoimmune inflammation of LG in SS, part of the clinical pathology of SS-associated dry eye, has not been a primary focus. Previous studies have shown that tear production is minimally affected by Restasis in SS patients,16 whereas many patients are either minimally responsive67 or unresponsive68 to this treatment. Clearly, additional therapeutic options that use simple topical eye drop administration of anti-inflammatory agents that have the ability to reduce autoimmune dacryoadenitis would be of clinical value to complement current therapies that are not effective in all patients.
Rapa is an FDA-approved immunomodulatory agent that binds with high affinity to the FK506 binding protein (FKBP12). The Rapa-FKBP12 complex is an allosteric inhibitor of mTOR, a kinase that plays a critical role in modulating both innate and adaptive immune responses. Additionally, mTOR mediates other activities, including regulation of the functions of antigen-presenting cells69 and also autophagy, a process that plays a key role in immunity and inflammation.70 Our previous study with intravenous Rapa demonstrated its ability to lower LG inflammation in male NOD mice with established disease,31 but due to its solubility issues, systemic administration of Rapa requires special formulation techniques to increase its solubility while mitigating its dose-limiting toxicity. We chose, in this study, to explore the therapeutic effects of Rapa eye drops on disease starting with early onset of symptoms through established disease, knowing that intravenous Rapa has the potential to suppress LG inflammation in mice with established disease.31 Further studies will explore the extent to which Rapa eye drops may mitigate well-established SS disease by reducing lymphocytic infiltration, increasing tear flow and restoring normal tear protein secretory profiles. Likewise, future studies may explore the possibility that a therapeutic effect on either LG inflammation or tear flow may occur before 12 weeks and 6 weeks, respectively, of Rapa eye drop treatment as used in our study.
To our knowledge, although ophthalmologic solutions of Rapa have been used previously for other indications,71,72 this is the first study of its effect on LG inflammation and aqueous tear deficiency associated with SS. A recent study, administering Rapa in a different phospholipid formulation by subconjunctival injection to dogs with keratoconjunctivitis sicca, showed an improvement in tear production and ocular surface integrity; however, the in vivo studies of this formulation were limited in scope and warrant further testing.73 A few studies have been reported for treatment of dry eye with topical tacrolimus (FK-506), a related inhibitor of mTOR, in mouse models as well as in humans, demonstrating an improvement in ocular surface staining, tear production, and a reduction in inflammatory cytokines at the ocular surface as well as in the LG.74–76 An expedited path to the clinic could be identified for Rapa eye drops for treatment of autoimmune dacryoadenitis in SS, because local delivery in our study showed comparable reduction of autoimmune inflammation of LG and other symptoms of SS-associated dry eye.
Sjögren's syndrome is characterized by lymphocytic infiltration of the exocrine glands, lacrimal and salivary, that are responsible for the production and release of the fluid and proteins essential for health of the ocular surface and oral cavity, respectively. This destruction of acinar cells in the LG is accompanied by impaired tear secretion and dry eye symptoms.77,78 Previous studies lend strong support to the notion that increased tear secretion is possible with suppression of LG inflammation,7,8 and in line with that, the primary objective of this study was to determine whether topical Rapa could reduce LG inflammation as well as improve tear flow and ocular surface integrity. Our findings reveal that a regimen of 12 weeks of twice-daily topical Rapa, starting at 8 weeks of age and ending at 20 weeks of age, in male NOD mice significantly reduced lymphocytic infiltration in the LG. This regimen also, at least for CTSS, reduced the activity of this putative SS biomarker in tears and specifically in the tear-producing LG acinar cells, suggesting the ability of Rapa to modulate protein expression and secretion in LG acinar cells, in addition to simply improving tear flow. Finally, after 12 weeks of twice-daily treatment, Rapa eye drops significantly lowered corneal staining relative to vehicle, indicating improvement in corneal barrier integrity.
The exact mechanism by which topical Rapa lowers inflammation in the LG and/or reduces tear flow was not the major focus of our study, but it may be related to the documented ability of Rapa to reduce T-cell proliferation by inhibiting mTOR seen in other tissues.35 Here we show that Rapa administered in eye drops reaches the LG as well as the draining lymph nodes and the systemic circulation in low concentrations. Thus, it is plausible that the effect of Rapa on infiltration and secretion maybe be due to modulation of the LG acinar cells and/or of modulation of lymphocytes locally in both the LG and draining lymph nodes. Certainly, many of the changes in LG gene expression associated with Rapa eye drops are consistent with those observed previously for intravenous Rapa administration, including reduced expression of autophagy-related and inflammation-associated genes.
Studies conducted by our laboratory have shown a prominent increase in CTSS activity in NOD mouse tears and LG,29 which was recently confirmed in tears from SS patients,30 suggesting its increased activity as a tear biomarker for diagnosis of SS. Additionally, recent evidence has also shown that elevation of CTSS in the salivary gland may perpetuate autoimmune responses by activating MHCII-mediated antigen presentation.63 In addition to reduced lymphocytic infiltration of the LG, male NOD mice treated with topical Rapa for 12 weeks showed decreased tear and LG CTSS activity, consistent with a role for this protease in mediation of inflammation in the LG. Preliminary studies in our laboratory have shown that CTSS expression and activity in the LG increases at the same time as the onset of infiltration (data not shown); however, it is not yet known if the elevation of CTSS triggers infiltration or if the activity increases due to increased infiltration. Here, we showed that the reduction of CTSS expression observed in the LG of Rapa-treated mice occurred primarily in the acinar cells, which may point toward the first notion.
In a previous study in NOD mice LG, we showed that inflamed LG in these mice exhibited increased gene expression of IFN-γ, TNF-α, and IL-12a,29 proinflammatory mediators of pathogenesis in SS.79,80 With the 12-week Rapa treatment relative to vehicle, the expression of these cytokines was significantly decreased in NOD mouse LG. MHCII, involved in antigen presentation and implicated in SS-associated dry eye, was also significantly decreased by drug treatment. Finally, reduced expression of Akt3, implicated in autophagy, which contributes to autoimmunity and inflammation, was exerted in the LG by 12-week Rapa treatment. Taken together, these data suggest that Rapa eye drops exert a potent anti-inflammatory effect in the LG.
In summary, our work has demonstrated that Rapa may be administered in an eye drop formulation to reduce autoimmune inflammation of the tear-secreting LG, increase tear secretion, and restore ocular surface homeostasis in a model of SS. The formulation was well tolerated when administered over 12 weeks in an established murine model of SS that recapitulates several characteristics of SS-associated dry eye in patients. Further studies with this ocular formulation of Rapa will be required to clearly understand the precise molecular mechanisms responsible for its immunomodulatory effect in the LG.
Supplementary Material
Acknowledgments
The authors thank Jingwen Chen for her contributions toward the experimental design. The Translational Research Laboratory at the USC School of Pharmacy also supported this work. Histology services were provided by the Cell and Tissue Imaging Core of the USC Research Center for Liver Diseases (National Institutes of Health [NIH] P30 DK-48522).
Supported by NIH Grant R01 EY011386 (SHA); an unrestricted departmental grant from Research to Prevent Blindness, New York, NY, USA; and a pilot grant from the Southern California-Clinical and Translational Science Institute (CD), supported by NIH UL1TR001855.
Disclosure: M. Shah, None; M.C. Edman, None; S. Reddy Janga, None; F. Yarber, None; Z. Meng, None; W. Klinngam, None; J. Bushman, None; T. Ma, None; S. Liu, None; S. Louie, None; A. Mehta, None; C. Ding, None; J.A. MacKay, None; S.F. Hamm-Alvarez, None
References
- 1. Lemp MA. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007; 5: 75–92. [DOI] [PubMed] [Google Scholar]
- 2. Rocha EM,, Alves M,, Rios JD,, Dartt DA. The aging lacrimal gland: changes in structure and function. Ocul Surf. 2008; 6: 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bayetto K,, Logan RM. Sjogren's syndrome: a review of aetiology, pathogenesis, diagnosis and management. Aust Dent J. 2010; 55: 39–47. [DOI] [PubMed] [Google Scholar]
- 4. Liu Z,, Pflugfelder SC. Corneal surface regularity and the effect of artificial tears in aqueous tear deficiency. Ophthalmology. 1999; 106: 939–943. [DOI] [PubMed] [Google Scholar]
- 5. Goto E,, Yagi Y,, Matsumoto Y,, Tsubota K. Impaired functional visual acuity of dry eye patients. Am J Ophthalmol. 2002; 133: 181–186. [DOI] [PubMed] [Google Scholar]
- 6. Humphreys-Beher MG,, Brayer J,, Yamachika S,, Peck AB,, Jonsson R. An alternative perspective to the immune response in autoimmune exocrinopathy: induction of functional quiescence rather than destructive autoaggression. Scand J Immunol. 1999; 49: 7–10. [DOI] [PubMed] [Google Scholar]
- 7. Nishiyama T,, Mishima K,, Obara K,, et al. Amelioration of lacrimal gland inflammation by oral administration of K-13182 in Sjögren's syndrome model mice. Clin Exp Immunol. 2007; 149: 586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsubota K,, Fujita H,, Tadano K,, et al. Improvement of lacrimal function by topical application of CyA in murine models of Sjogren's syndrome. Invest Ophthalmol Vis Sci. 2001; 42: 101–110. [PubMed] [Google Scholar]
- 9. Foulks GN,, Forstot SL,, Donshik PC,, et al. Clinical guidelines for management of dry eye associated with Sjogren disease. Ocul Surf. 2015; 13: 118–132. [DOI] [PubMed] [Google Scholar]
- 10. Mavragani CP,, Nezos A,, Moutsopoulos HM. New advances in the classification, pathogenesis and treatment of Sjogren's syndrome. Curr Opin Rheumatol. 2013; 25: 623–629. [DOI] [PubMed] [Google Scholar]
- 11. Roberts CW,, Carniglia PE,, Brazzo BG. Comparison of topical cyclosporine, punctal occlusion, and a combination for the treatment of dry eye. Cornea. 2007; 26: 805–809. [DOI] [PubMed] [Google Scholar]
- 12. Kymionis GD,, Bouzoukis DI,, Diakonis VF,, Siganos C. Treatment of chronic dry eye: focus on cyclosporine. Clin Ophthalmol. 2008; 2: 829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liew SH,, Nichols KK,, Klamerus KJ,, Li JZ,, Zhang M,, Foulks GN. Tofacitinib (CP-690,550), a Janus kinase inhibitor for dry eye disease: results from a phase 1/2 trial. Ophthalmology. 2012; 119: 1328–1335. [DOI] [PubMed] [Google Scholar]
- 14. Sall K,, Stevenson OD,, Mundorf TK,, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000; 107: 631–639. [DOI] [PubMed] [Google Scholar]
- 15. Stevenson D,, Tauber J,, Reis BL. Efficacy and safety of cyclosporin A ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: a dose-ranging, randomized trial. The Cyclosporin A Phase 2 Study Group. Ophthalmology. 2000; 107: 967–974. [DOI] [PubMed] [Google Scholar]
- 16. Hyon JY,, Lee YJ,, Yun PY. Management of ocular surface inflammation in Sjogren syndrome. Cornea. 2007; 26: S13–S15. [DOI] [PubMed] [Google Scholar]
- 17. de Paiva CS,, Pflugfelder SC. Rationale for anti-inflammatory therapy in dry eye syndrome. Arq Bras Oftalmol. 2008; 71: 89–95. [DOI] [PubMed] [Google Scholar]
- 18. The Medical Letter, Inc. Lifitegrast (Xiidra) for dry eye disease. Med Lett Drugs Ther. 2016; 58: 110–111. [PubMed] [Google Scholar]
- 19. Tauber J,, Karpecki P,, Latkany R,, et al. Lifitegrast ophthalmic solution 5.0% versus placebo for treatment of dry eye disease: results of the randomized phase III OPUS-2 study. Ophthalmology. 2015; 122: 2423–2431. [DOI] [PubMed] [Google Scholar]
- 20. Semba CP,, Torkildsen GL,, Lonsdale JD,, et al. A phase 2 randomized, double-masked, placebo-controlled study of a novel integrin antagonist (SAR 1118) for the treatment of dry eye. Am J Ophthalmol. 2012; 153: 1050–1060.e1. [DOI] [PubMed] [Google Scholar]
- 21. Jung HH,, Ji YS,, Sung MS,, Kim KK,, Yoon KC. Long-term outcome of treatment with topical corticosteroids for severe dry eye associated with Sjogren's syndrome. Chonnam Med J. 2015; 51: 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. da Costa SR,, Wu K,, Veigh MM,, et al. Male NOD mouse external lacrimal glands exhibit profound changes in the exocytotic pathway early in postnatal development. Exp Eye Res. 2006; 82: 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chiorini JA,, Cihakova D,, Ouellette CE,, Caturegli P. Sjogren syndrome: advances in the pathogenesis from animal models. J Autoimmun. 2009; 33: 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schenke-Layland K,, Xie J,, Angelis E,, et al. Increased degradation of extracellular matrix structures of lacrimal glands implicated in the pathogenesis of Sjogren's syndrome. Matrix Biol. 2008; 27: 53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lindqvist AK,, Nakken B,, Sundler M,, et al. Influence on spontaneous tissue inflammation by the major histocompatibility complex region in the nonobese diabetic mouse. Scand J Immunol. 2005; 61: 119–127. [DOI] [PubMed] [Google Scholar]
- 26. Robinson CP,, Cornelius J,, Bounous DI,, Yamamoto H,, Humphreys-Beher MG,, Peck AB. Infiltrating lymphocyte populations and cytokine production in the salivary and lacrimal glands of autoimmune NOD mice. Adv Exp Med Biol. 1998; 438: 493–497. [DOI] [PubMed] [Google Scholar]
- 27. Yamano S,, Atkinson JC,, Baum BJ,, Fox PC. Salivary gland cytokine expression in NOD and normal BALB/c mice. Clin Immunol. 1999; 92: 265–275. [DOI] [PubMed] [Google Scholar]
- 28. Mikulowska-Mennis A,, Xu B,, Berberian JM,, Michie SA. Lymphocyte migration to inflamed lacrimal glands is mediated by vascular cell adhesion molecule-1/α(4)β(1) integrin, peripheral node addressin/L-selectin, and lymphocyte function-associated antigen-1 adhesion pathways. Am J Pathol. 2001; 159: 671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li X,, Wu K,, Edman M,, et al. Increased expression of cathepsins and obesity-induced proinflammatory cytokines in lacrimal glands of male NOD mouse. Invest Ophthalmol Vis Sci. 2010; 51: 5019–5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hamm-Alvarez SF,, Janga SR,, Edman MC,, et al. Tear cathepsin S as a candidate biomarker for Sjogren's syndrome. Arthritis Rheumatol. 2014; 66: 1872–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shah M,, Edman MC,, Janga SR,, et al. A rapamycin-binding protein polymer nanoparticle shows potent therapeutic activity in suppressing autoimmune dacryoadenitis in a mouse model of Sjogren's syndrome. J Control Release. 2013; 171: 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thomson AW,, Turnquist HR,, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009; 9: 324–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chan S. Targeting the mammalian target of rapamycin (mTOR): a new approach to treating cancer. Br J Cancer. 2004; 91: 1420–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mayer DF,, Kushwaha SS. Transplant immunosuppressant agents and their role in autoimmune rheumatic diseases. Curr Opin Rheumatol. 2003; 15: 219–225. [DOI] [PubMed] [Google Scholar]
- 35. Carlson RP,, Baeder WL,, Caccese RG,, Warner LM,, Sehgal SN. Effects of orally administered rapamycin in animal models of arthritis and other autoimmune diseases. Ann N Y Acad Sci. 1993; 685: 86–113. [DOI] [PubMed] [Google Scholar]
- 36. Boers-Doets CB,, Raber-Durlacher JE,, Treister NS,, et al. Mammalian target of rapamycin inhibitor-associated stomatitis. Future Oncol. 2013; 9: 1883–1892. [DOI] [PubMed] [Google Scholar]
- 37. Marti HP,, Frey FJ. Nephrotoxicity of rapamycin: an emerging problem in clinical medicine. Nephrol Dial Transplant. 2005; 20: 13–15. [DOI] [PubMed] [Google Scholar]
- 38. Banhara PB,, Goncalves RT,, Rocha PT,, Delgado AG,, Leite M, Jr,, Gomes CP. Tubular dysfunction in renal transplant patients using sirolimus or tacrolimus. Clin Nephrol. 2015; 83: 331–337. [DOI] [PubMed] [Google Scholar]
- 39. Lopez P,, Kohler S,, Dimri S. Interstitial lung disease associated with mTOR inhibitors in solid organ transplant recipients: results from a large phase III clinical trial program of everolimus and review of the literature. J Transplant. 2014; 2014: 305931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shah M,, Hsueh PY,, Sun G,, Chang HY,, Janib SM,, MacKay JA. Biodegradation of elastin-like polypeptide nanoparticles. Protein Sci. 2012; 21: 743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tsubota K,, Saito I,, Ishimaru N,, Hayashi Y. Use of topical cyclosporin A in a primary Sjogren's syndrome mouse model. Invest Ophthalmol Vis Sci. 1998; 39: 1551–1559. [PubMed] [Google Scholar]
- 42. Simamora P,, Alvarez JM,, Yalkowsky SH. Solubilization of rapamycin. Int J Pharm. 2001; 213: 25–29. [DOI] [PubMed] [Google Scholar]
- 43. Leonardi A,, Bucolo C,, Romano GL,, et al. Influence of different surfactants on the technological properties and in vivo ocular tolerability of lipid nanoparticles. Int J Pharm. 2014; 470: 133–140. [DOI] [PubMed] [Google Scholar]
- 44. Oh JY,, Yu JM,, Ko JH. Analysis of ethanol effects on corneal epithelium. Invest Ophthalmol Vis Sci. 2013; 54: 3852–3856. [DOI] [PubMed] [Google Scholar]
- 45. Sun G,, Hsueh PY,, Janib SM,, Hamm-Alvarez S, Andrew MacKay J. Design and cellular internalization of genetically engineered polypeptide nanoparticles displaying adenovirus knob domain. J Control Release. 2011; 155: 218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aluri S,, Pastuszka MK,, Moses AS,, MacKay JA. Elastin-like peptide amphiphiles form nanofibers with tunable length. Biomacromolecules. 2012; 13: 2645–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lemp MA. Report of the National Eye Institute/Industry Workshop on Clinical Trials in Dry Eyes. CLAO J. 1995; 21: 221–232. [PubMed] [Google Scholar]
- 48. Turpie B,, Yoshimura T,, Gulati A,, Rios JD,, Dartt DA,, Masli S. Sjogren's syndrome-like ocular surface disease in thrombospondin-1 deficient mice. Am J Pathol. 2009; 175: 1136–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ding C,, Parsa L,, Nandoskar P,, Zhao P,, Wu K,, Wang Y. Duct system of the rabbit lacrimal gland: structural characteristics and role in lacrimal secretion. Invest Ophthalmol Vis Sci. 2010; 51: 2960–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wu K,, Joffre C,, Li X,, et al. Altered expression of genes functioning in lipid homeostasis is associated with lipid deposition in NOD mouse lacrimal gland. Exp Eye Res. 2009; 89: 319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sezgin Z,, Yuksel N,, Baykara T. Preparation and characterization of polymeric micelles for solubilization of poorly soluble anticancer drugs. Eur J Pharm Biopharm. 2006; 64: 261–268. [DOI] [PubMed] [Google Scholar]
- 52. Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007; 24: 1–16. [DOI] [PubMed] [Google Scholar]
- 53. Kaila T,, Huupponen R,, Salminen L. Effects of eyelid closure and nasolacrimal duct occlusion on the systemic absorption of ocular timolol in human subjects. J Ocul Pharmacol. 1986; 2: 365–369. [DOI] [PubMed] [Google Scholar]
- 54. Lee YH,, Kompella UB,, Lee VH. Systemic absorption pathways of topically applied beta adrenergic antagonists in the pigmented rabbit. Exp Eye Res. 1993; 57: 341–349. [DOI] [PubMed] [Google Scholar]
- 55. Schenke-Layland K,, Xie J,, Magnusson M,, et al. Lymphocytic infiltration leads to degradation of lacrimal gland extracellular matrix structures in NOD mice exhibiting a Sjogren's syndrome-like exocrinopathy. Exp Eye Res. 2010; 90: 223–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Doyle ME,, Boggs L,, Attia R,, et al. Autoimmune dacryoadenitis of NOD/LtJ mice and its subsequent effects on tear protein composition. Am J Pathol. 2007; 171: 1224–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Papiris SA,, Maniati M,, Constantopoulos SH,, Roussos C,, Moutsopoulos HM,, Skopouli FN. Lung involvement in primary Sjogren's syndrome is mainly related to the small airway disease. Ann Rheum Dis. 1999; 58: 61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Parambil JG,, Myers JL,, Lindell RM,, Matteson EL,, Ryu JH. Interstitial lung disease in primary Sjogren syndrome. Chest. 2006; 130: 1489–1495. [DOI] [PubMed] [Google Scholar]
- 59. Matsumoto T,, Morizane T,, Aoki Y,, et al. Autoimmune hepatitis in primary Sjogren's syndrome: pathological study of the livers and labial salivary glands in 17 patients with primary Sjogren's syndrome. Pathol Int. 2005; 55: 70–76. [DOI] [PubMed] [Google Scholar]
- 60. Kikutani H,, Makino S. The murine autoimmune diabetes model: NOD and related strains. Adv Immunol. 1992; 51: 285–322. [DOI] [PubMed] [Google Scholar]
- 61. Bao M,, Yang Y,, Jun HS,, Yoon JW. Molecular mechanisms for gender differences in susceptibility to T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Immunol. 2002; 168: 5369–5375. [DOI] [PubMed] [Google Scholar]
- 62. Morris MA,, McDuffie M,, Nadler JL,, Ley K. Prevention, but not cure, of autoimmune diabetes in a NOD.scid transfer model by FTY720 despite effective modulation of blood T cells. Autoimmunity. 2011; 44: 115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Saegusa K,, Ishimaru N,, Yanagi K,, et al. Cathepsin S inhibitor prevents autoantigen presentation and autoimmunity. J Clin Invest. 2002; 110: 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Morgan-Bathke M,, Lin HH,, Ann DK,, Limesand KH. The role of autophagy in salivary gland homeostasis and stress responses. J Dent Res. 2015; 94: 1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Corum DG,, Tsichlis PN,, Muise-Helmericks RC. AKT3 controls mitochondrial biogenesis and autophagy via regulation of the major nuclear export protein CRM-1. FASEB J. 2014; 28: 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Alers S,, Loffler AS,, Wesselborg S,, Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol. 2012; 32: 2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dastjerdi MH,, Hamrah P,, Dana R. High-frequency topical cyclosporine 0.05% in the treatment of severe dry eye refractory to twice-daily regimen. Cornea. 2009; 28: 1091–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Williamson JF,, Huynh K,, Weaver MA,, Davis RM. Perceptions of dry eye disease management in current clinical practice. Eye Contact Lens. 2014; 40: 111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Saric A,, Hipolito VE,, Kay JG,, Canton J,, Antonescu CN,, Botelho RJ. mTOR controls lysosome tubulation and antigen presentation in macrophages and dendritic cells. Mol Biol Cell. 2015; 27: 321–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Benjamin D,, Colombi M,, Moroni C,, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011; 10: 868–880. [DOI] [PubMed] [Google Scholar]
- 71. Bhatt N,, Dalal M,, Tucker W,, Obiyor D,, Nussenblatt R,, Sen HN. Subconjunctival sirolimus in the treatment of autoimmune non-necrotizing anterior scleritis: results of a phase I/II clinical trial. Am J Ophthalmol. 2015; 159: 601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Eren K,, Turgut B,, Akin MM,, Demir T. The suppression of wound healing response with sirolimus and sunitinib following experimental trabeculectomy in a rabbit model. Curr Eye Res. 2015; 1–10. [DOI] [PubMed]
- 73. Linares-Alba MA,, Gomez-Guajardo MB,, Fonzar JF,, Brooks DE,, Garcia-Sanchez GA,, Bernad-Bernad MJ. Preformulation studies of a liposomal formulation containing sirolimus for the treatment of dry eye disease. J Ocul Pharmacol Ther. 2016; 32: 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Moscovici BK,, Holzchuh R,, Sakassegawa-Naves FE,, et al. Treatment of Sjogren's syndrome dry eye using 0.03% tacrolimus eye drop: prospective double-blind randomized study. Cont Lens Anterior Eye. 2015; 38: 373–378. [DOI] [PubMed] [Google Scholar]
- 75. Lin BW,, Chen MZ,, Fan SX,, Chuck RS,, Zhou SY. Effect of 0.025% FK-506 eyedrops on botulinum toxin B-induced mouse dry eye. Invest Ophthalmol Vis Sci. 2015; 56: 45–53. [DOI] [PubMed] [Google Scholar]
- 76. Sanz-Marco E,, Udaondo P,, Garcia-Delpech S,, Vazquez A,, Diaz-Llopis M. Treatment of refractory dry eye associated with graft versus host disease with 0.03% tacrolimus eyedrops. J Ocul Pharmacol Ther. 2013; 29: 776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lemp MA. Dry eye (Keratoconjunctivitis sicca), rheumatoid arthritis, and Sjogren's syndrome. Am J Ophthalmol. 2005; 140: 898–899. [DOI] [PubMed] [Google Scholar]
- 78. Saleh J,, Figueiredo MA,, Cherubini K,, Salum FG. Salivary hypofunction: an update on aetiology, diagnosis and therapeutics. Arch Oral Biol. 2015; 60: 242–255. [DOI] [PubMed] [Google Scholar]
- 79. Lessard CJ,, Li H,, Adrianto I,, et al. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjogren's syndrome. Nat Genet. 2013; 45: 1284–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nocturne G,, Mariette X. Advances in understanding the pathogenesis of primary Sjogren's syndrome. Nat Rev Rheumatol. 2013; 9: 544–556. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.