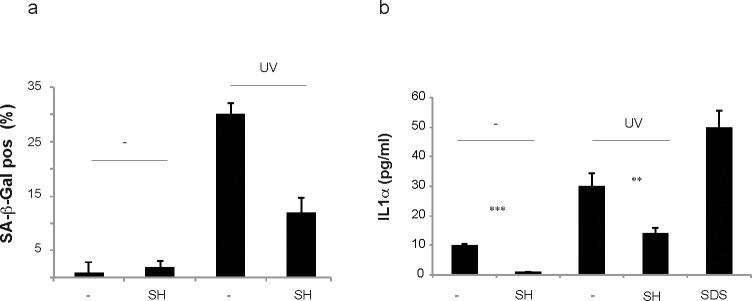
Figure 6. Toxicity and irritability evaluation of S. haenkei extract in reconstituted human epidermis.
Skin issues were cultured in 12 well plates containing 37°C pre-warmed maintenance media (2 ml/well) and incubated overnight at 37°C, 5% CO2 and 95% humidity, prior to the experiment. EpiSkin tissues were irradiated with UVB (30J/m2) and 3 hours later treated by topical application with 10μg/ml Salvia haenkei extract and 5% SDS for positive control. 4h later, the epidermis was washed with PBS and left for incubation at 37°C, 5% CO2. (a) 22h after topical application of 10μg/ml of SH on EpiSkin tissues, senescence (bars) was calculated as a percentage of the control for β-galactosidase positive cells. Here, the treatment with UV was used as positive control. Results are expressed as the mean (+SEM) of triplicates in one representative experiment. (b) IL1ɑ production by EpiSkin tissue in response to S. haenkei treatment in the presence or absence of UV irradiation. Treatment with SDS was used as positive control. 22h after topical application of 10μg/ml of S. haenkei extract on EpiSkin tissues, supernatants were collected and samples stored at −80°C. The levels of IL1α were tested by ELISA. Results are represented in logarithmic values (pg/ml) and expressed as mean value±SEM, from triplicates in one experiment.