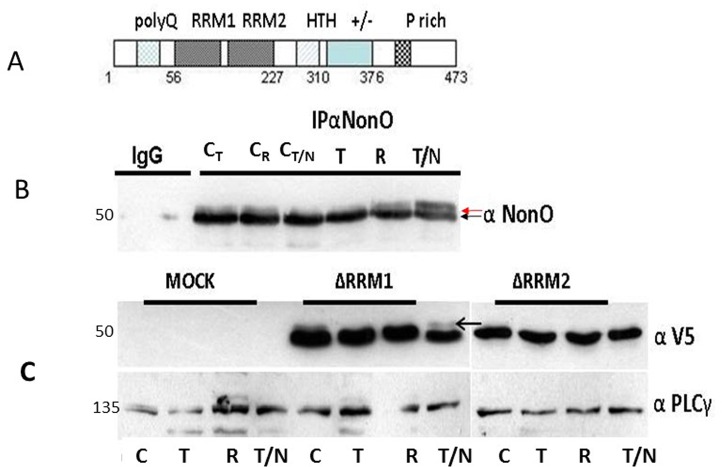
Figure 5. Posttranslational modification of NonO during mitosis.
(A) Schematic of NonO domains. polyQ, polyglutamine tract; RRM1 and 2, RNA Recognition Motifs; HTH, predicted helix-turn-helix domain; +/−, highly charged region; P, proline-rich. (B) The molecular weight of NonO (50 kD) is increased by post-translational modification at G2/M. Cells were lysed after 16 hr thymidine (T) pulse, then released for 8h (R) prior to incubation for 16h in nocodazole (N/T). NonO was immunoprecipitated with mouse anti (α)-NonO mAb and detected by Western blotting with rabbit polyclonal α-NonO; mouse IgG served as negative control. As controls, non-synchronized cells were lysed at the indicated time points in the same buffer (CT: 16 h thymidine; CR: 8h release; CN/T: 16 h nocodazole). A doublet is observed in control, but not in G2/M arrested cells. Modified NonO levels (upper arrow) increase between G1/S (thymidine-arrested) and G2/M (nocodazole-arrested) phases. (C) The NonO RRM2 domain is targeted by cell cycle-dependent modification. NonO deletion (Δ) mutants were analyzed under lysate and treatment conditions described above. The construction of NonO ΔRRM1 (lacking residues 87-160) and ΔRRM2 (lacking residues 160-227) is detailed in Materials and Methods. Protein loads in Western analyses were confirmed by anti-PLCγ (135 kD). NonO-deletion mutants were detected via their V5-N-terminal tags.