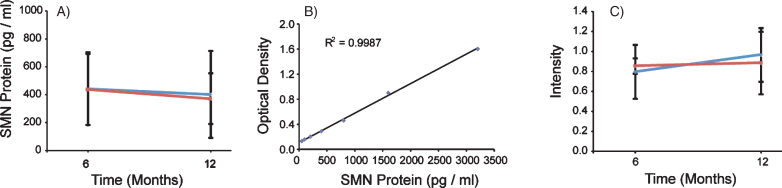
Fig.5.
SMN protein concentrations in PBMCs were not altered in the presence of VPA or Placebo. SMN protein was measured using the SMN ELISA and absorbance values were extrapolated from the provided standard curve. A) Both Group 1 and Group 2 mean SMN protein levels were calculated by visit, and 13 and 18 subjects respectively. Group 1 (VPA/Placebo) had mean SMN protein concentrations of 442.8 (±260.7) pg per ml of total protein to 402.39 (±311.5) pg/ml of total protein. Group 2 (Placebo/VPA) had similar concentrations of SMN protein, 438.1 (±254.3) to 372.2 (±182.1) pg/ml of total protein, p > 0.05. B) Representative standard curve from one of the four plates ran for this experiment. Purified SMN protein was loaded on the plate by a serial dilution from 50 pg/ml to 3200 pg/ml, these concentrations correspond to optical densities yielding a linear regression with an r2 value of 0.9987. C) SMN Cell Immunoassay. SMN protein concentrations were standardized by taking a ratio of SMN protein to that of an endogenous protein control, Y12. Group 1 (VPA/Placebo) had an N of 9 and the mean ratios of SMN/Y12 were 0.796 (±0.077) to 0.965 (±0.313) Group 2 (Placebo/VPA) had a 0.965 (±0.313). Group 2 (Placebo/VPA) had an N of 12 and the mean ratios of SMN/Y12 were 0.854 (±0.269) to 0.884 (±0.268), p > 0.05.