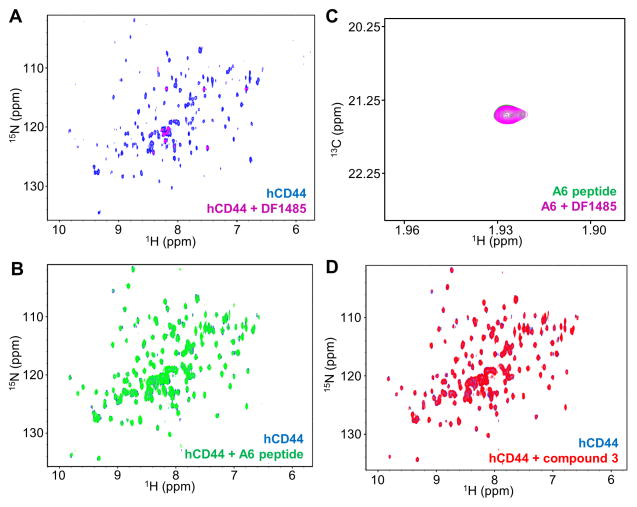
Figure 2. Biophysical validation of hCD44 putative binders.
(A) CD44 antibody DF1485. 2D [1H,15N]-sofast-HMQC spectra of 10 μM 15N-hCD44(21–178) recorded in absence (blue) and in presence (magenta) of 10 μM DF1485 antibody. Due to the high molecular weight of the antibody-CD44 complex, as a result of the binding, the signals of the protein are broadened beyond detection. After the addition of 500 μM A6 peptide the antibody is not displaced, corroborating the findings of panel B that A6-peptide does not appreciably bind to hCD44(21–178).
A6-peptide. (B) 2D [1H,15N]-sofast-HMQC spectra of 20 μM 15N-hCD44(21–178) in absence (blue) and in presence (green) of 500 μM A6 peptide. No appreciable binding is detected under those experimental conditions. (C) 2D [1H,13C]-HSQC spectra of 5 μM 13C-A6 peptide in absence (green) and in presence (magenta) of 4 μM DF1485 antibody. The signal of the 13C-labeled acetyl methyl of the peptide is not perturbed by the presence of the antibody, indicating the absence of significant interactions between the A6-peptide and the antibody.
(D) Compound 3[16]. 2D [1H,15N]-sofast-HMQC spectra of 20 μM 15N-hCD44(21–178) recorded in absence (blue) and in presence (red) of 2 mM compound 3 [16]. No binding is detected. The absence of significant interactions is confirmed by Isothermal Titration Calorimetry in a competition assay in which hCD44(21–178) is incubated with a 750-fold molar excess of compound 3 and subsequently the binding of HA8 is probed. The Kd detected for HA8 under this condition was 37.5 μM with a ΔH= −0.83 Kcal/mol, hence not significantly different than the binding of HA8 to hCD44(21–178) in absence of compound 3 (Supplementary Figure S3).