Table 1.
| Putative CD44 binders | References and comments | |||
|---|---|---|---|---|
| Natural Ligand | ||||
| HA8 : [GlcNAc-GlcUA]4 | [21] [4a] [4b] [20] | Kd = 24.6 μM by ITCa | ||
| Antibodies | ||||
| DF1485 | [25] | From Santa Cruz Biotechnolgy Kd ≪ 400 nM by NMRb |
||
| Roche | Patent Number: WO 2011095498 A1c | |||
| Peptides | ||||
| A6 Peptide: Ac-KPSSPPEE-NH2 | [25] [9] | In phase 2 for CLL |
|
No direct bind to recombinant hCD44(21–178) by NMRd |
| A5G27: H-RLVSYNGIIFFLK-NH2 | [26] | Laminin α5 peptide | ||
| Ac-SRPQGPFL-NH2 | [28] | From blade I of MMP-9 | ||
| Fragment | ||||
Compound 3:
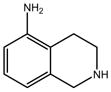
|
[16] | Reported Kd by SPR is 0.9 mM No appreciable binding by NMR or ITCe |
||
Isothermal Titration Calorimetry (ITC) data as reported in Figure 1. The data are in agreement with similar measurements from ref. [17].
No Kd was reported in literature for this antibody. By NMR spectroscopy with recombinant 15N-hCD44(21–178) we estimated a Kd value ≪ 400 nM.
This antibody is not commercially available. We tested peptides regions cited in the patent as putative CD44 binding elements. However, under the reported experimental condition, none of these peptides bound to hCD44(21–178) significantly.
Using 15N-hCD44(21–178) at 20 μM we could not detect significant binding for these peptides when tested at a concentration up to 500 μM.
In [1H,15N]-sofast-HMQC correlation spectra with 15N-hCD44(21–178) at 20 μM, compound 3 did not show appreciable binding up to 20 mM. In addition, the compound was not able to significantly displace the binding of HA8 to CD44 at 55 mM by ITC (see text and supporting information).
