Abstract
Omega-3 fatty acid administration can affect the release of neurotransmitters and reduce inflammation and oxidative stress, but its use in traumatic brain injury (TBI) has not been described extensively. We investigated the effect of 7 day oral fish oil treatment in the recovery of potassium evoked dopamine release after TBI. Sham rats and TBI rats were given either olive oil or fish oil by oral gavage and were subject to cerebral microdialysis. Olive oil treated TBI rats showed significant dopamine release deficit compared to sham rats, and this deficit was restored with dietary omega-3 fatty acids. There was no effect of fish oil treatment on extracellular levels of dopamine metabolites such as 3,4-dihydroxyphenylacetic acid and homovanillic acid. These results suggest the therapeutic potential of omega-3 fatty acids in restoring dopamine neurotransmission deficits after TBI.
Keywords: fish oil, omega-3 fatty acid, docosahexaenoic acid, eicosapentaenoic acid, traumatic brain injury, dopamine
Introduction
Polyunsaturated fatty acids (PUFA) such as omega-6 and omega-3 fatty acids are major components of neuronal membranes of the brain. They are obtained exclusively by diet, and their deprivation can lead to performance deficits in learning [32]. Their health benefits have long been investigated in cardiovascular and chronic inflammatory diseases. But more recently, there has been an increasing interest in the role of omega-3 fatty acids such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) in diseases of the central nervous system. Studies have shown the involvement of omega-3 fatty acids in psychiatric disorders such as depression and schizophrenia [28], neurodegenerative diseases such as Alzheimer’s disease [12] and Parkinson’s disease [8], and acute brain injury such as ischemia [11]. Also, a few recent studies have demonstrated the possibility of using omega-3 fatty acids as a therapeutic strategy post TBI by showing reduction in amyloid precursor protein and caspase-3, markers of axonal injury and apoptosis, respectively [4,25].
Dietary intake of omega-3 fatty acids have also been shown to affect the release of neurotransmitters. Previous microdialysis experiments demonstrated that dietary DHA deficiency reduces potassium evoked acetylcholine release in the hippocampus [2], whereas dietary enrichment of DHA increases it [17,3]. Similarly, omega-3 fatty acid deficient diet was shown to reduce tyramine stimulated dopamine release in the frontal cortex and nucleus accumbens [37,39], but dietary enrichment of it during early developmental stage was shown to restore dopamine release [22]. Electron micrographs revealed that rats with omega-3 fatty acid deficient diet had significantly decreased number of dopamine vesicles, suggesting that sufficient omega-3 fatty acid intake is needed for normal dopamine neurotransmission in the brain [38].
In an animal model of Parkinson’s disease using 6-hydroxydopamine, chronic dietary DHA supplementation resulted in attenuation of apomorphine induced rotating behavior in rats and lipid peroxidation [14]. Similarly, in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model of Parkinson’s disease, oral intake of DHA was shown to have neuroprotective effects for dopamine neurons of substantia nigra and reduce motor deficits [31]. In this study by Tanriover et al., DHA was shown to increase expression of glial cell line-derived neurotrophic factor and neurturin. Also, omega-3 fatty acid diet consisting of both DHA and EPA increased brain derived neurotrophic factor (BDNF) mRNA expression in the striatum and BDNF protein levels in the motor cortex [9]. Since these neurotrophic factors promote neural growth and development, their induction may be a possible mechanism of neuroprotection by omega-3 fatty acids.
In our recent work [30], we demonstrated reduced potassium evoked striatal dopamine release at 1 week post TBI, which may be partially responsible for functional deficits in animals after injury. Since dietary omega-3 fatty acid can affect the release of neurotransmitters, we hypothesized that TBI induced striatal dopamine release deficit may be attenuated by oral fish oil treatment post injury at the same time interval. This study demonstrates for the first time that post injury treatment with oral fish oil can restore a dopamine release deficit after TBI.
Material and methods
All experiments conducted in this study were approved by the Institutional Animal Care and Use Committee of University of Pittsburgh. Male Sprague-Dawley rats weighing 275-300g were housed in a controlled environment and given food and water ad libitum throughout the duration of the study. Animals were assigned to the following groups: sham + olive oil, sham + fish oil, injured + olive oil, and injured + fish oil (n=5-6 in each group).
Controlled cortical impact (CCI) model was used as previously described [15]. Briefly, animals were intubated and anesthesia was administered using 2% isoflurane and 2:1 N2O/O2 by mechanical ventilation throughout the duration of the surgery. After surgical incision and exposure of skull, craniectomy was performed over the right parietal bone. For groups receiving CCI, injury was given at 2.6mm deformation depth and 4m/s with the CCI device. For sham animals, only the craniectomy was performed. After CCI or sham injury, the animal recovery from anesthesia was observed by monitoring righting reflex.
After rats had full recovery from surgical procedures and anesthesia (5-6 minutes post surgery), animals were given commercially available laboratory purified fish oil (Nature’s Bounty) or olive oil as controls as used in previous human clinical trials [13,18]. The animals were given 1.5mL of fish oil or olive oil by oral gavage. The fish oil contained 360mg EPA and 240mg DHA. For the next 7 days, animals were given the same dose of oil every 24 hours via oral gavage.
Seven days post surgery, animals were re-anesthetized using 2% isoflurane and 2:1 N2O/O2. In vitro recovery of microdialysis probes was measured to be 24.2% at a flow rate of 2μl/min, with no statistically significant difference among the four groups. Dental drill was used to enlarge the craniotomy site for placement of microdialysis probe (SciPro, Sanborn, NY), which was implanted into the striatum ipsilateral to the site of injury (AP:+0.0mm, L:+2.8mm, DV:-4.0mm) and fixed using dental cement. The animals were then allowed to recover and were housed overnight in a Plexiglas chamber as previously described [30,16]. Artificial cerebrospinal fluid (ACSF) composed of 126.5mM NaCl, 2.4mM KCl, 1.1mM CaCl2, 0.83mM MgCl2, 27.5mM NaHCO3, and 0.5mM KH2PO4 was infused overnight at 0.2μl/min in awake, freely-moving animals. Next day, flow rate was increased to 2μl/min for 1 hour before the experiment. Samples were collected every 20 minutes during the experiment into Eppendorf tubes containing 5μL of 0.3M HClO2. Sixty minutes after initial sample collection began, ACSF was switched with high potassium ACSF solution containing 48.9mM NaCl, 80mM KCl, 1.1mM CaCl2, 0.83mM MgCl2, 27.5mM NaHCO3, and 0.5mM KH2PO4 with equal osmolarity as original ACSF. High potassium ACSF was switched back to original ACSF 40 minutes later. Following the microdialysis experiment, the rats were sacrificed and the locations of the probes were verified.
Dopamine and two of its major metabolites, 3,4-dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA) were measured in microdialysate samples using HPLC with CoulArray Detector (ESA, Chelmsford, MA, USA). The HPLC system was equipped with eight coulometric electrodes with potentials from −120 to +300 mV. Immediately after collection, microdialysates were analyzed and quantified. Baseline values of dopamine were below the linear range of detection and were thus reported as 0μM.
Statistical analysis was performed using one way ANOVA for the dopamine area under the curve for the four groups in this study. For DOPAC and HVA statistical analysis, baseline value for each animal was calculated by averaging the values at initial 3 collection times. Then, the magnitudes of deviation away from the baseline at each time point after the potassium stimulus were summed to calculate the area under the curve for each group. One way ANOVA was used also for DOPAC and HVA areas under the curve. When a one way ANOVA found significant between groups difference, a one-tailed Dunnett’s post-hoc analysis was used to compare each group to injured with olive oil treated group. Calculations were performed using PASW Statistics 19 software (SPSS Inc., Chicago, IL). Each data point was expressed as the group mean ± standard error of the mean.
Results
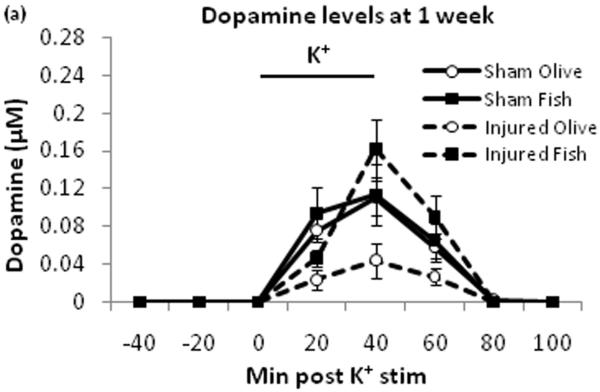
Potassium evoked extracellular dopamine for the injured + fish oil group showed lower dopamine levels compared to the three other groups (Fig. 1a). Dopamine peak areas under the curve were measured to assess the overall level of dopamine release in each group (Fig. 1b). There was a significant between-group difference with F3,17=3.591 (p≤0.05). Post-hoc test showed a significant difference between the injured + olive oil and each of the three other groups (p≤0.05).
Fig. 1.
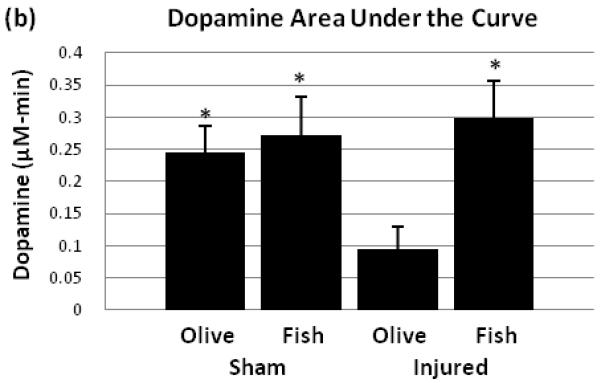
Potassium stimulated dopamine levels. Dopamine levels were monitored using microdialysis and HPLC. At 60 min after the initial sample collection began, potassium ACSF stimulus was given for 40 minutes (a). The areas under the curve for dopamine levels were compared among all four groups (b). Black bar represents times for potassium stimulus. SO = sham + olive oil, SF = sham + fish oil, IO = injured + olive oil, IF = injured + fish oil. * = p ≤ 0.05
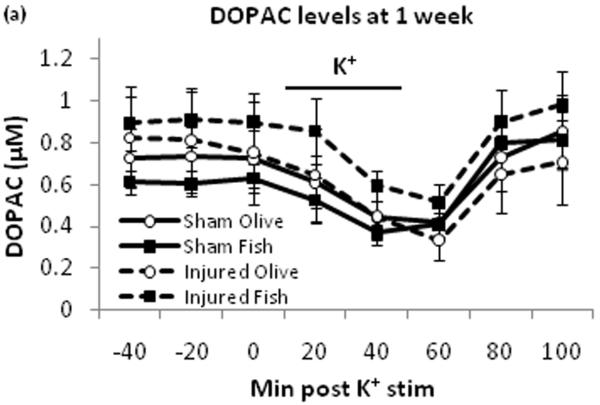
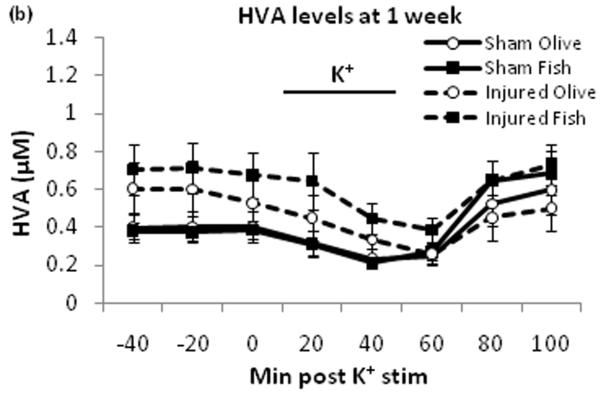
Potassium stimulus decreased extracellular DOPAC (Fig. 2a) and HVA (Fig. 2b) levels in all four groups. There was no significant between-group difference for the area under the curve for DOPAC and HVA [data not shown].
Fig. 2.
Extracellular DOPAC and HVA levels. DOPAC (a) and HVA (b) were monitored by HPLC. Potassium stimulus induced temporary decrease of DOPAC and HVA levels across all groups. Black bar represents times for potassium stimulus.
Discussion
This study demonstrates that TBI-induced deficits in evoked dopamine release in the striatum can be attenuated by daily oral treatment with fish oil. The effect of TBI in the olive oil control groups was consistent with our previous findings in which no treatments were given, showing dopamine release around 0.04μM with potassium stimulus [30]. This indicates that olive oil treatment has no effect on evoked dopamine release. There were no differences in extracellular DOPAC and HVA levels at one week post injury by fish oil treatment compared to olive oil treatment, suggesting that this treatment does not affect dopamine metabolizing enzymes such as monoamine oxidase and catechol-O-methyl transferase. Striatal dopamine neurotransmission has a major role in voluntary movement [23], motor learning [20], as well as spatial learning and memory [26]. Therefore, TBI induced deficit in striatal dopamine release may be a major contributing factor to behavioral impairments. The oral fish oil treatment’s reversal of dopamine release deficits may be a novel mechanism in the therapeutic use of omega-3 fatty acids after TBI.
Dietary treatment using omega-3 fatty acids may increase the synthesis of phosphatides which are major components of neuronal membranes. In a previous study, supplementing the diet of gerbils with DHA increased hippocampal dendritic spines, as well as synaptic proteins such as PSD-95 and syntaxin-1 [10,36]. While EPA also increased synaptic protein levels, arachidonic acid (omega-6 fatty acid) did not. Dietary supplementation using other precursors of phosphatides such as uridine [34] or cytidine (5′)diphosphocholine [1] increased the release of striatal dopamine. As previously mentioned, omega-3 fatty acid deficiency has been shown to reduce dopamine release [37], which may be due to reduced synaptic dopamine vesicles [38]. Because dietary enrichment of omega-3 fatty acid can restore dopamine release [22], dopamine vesicle formation by omega-3 fatty acid is a possible mechanism of restoring dopamine neurotransmission. In the current study, omega-3 fatty acid treatment following TBI may have promoted synaptogenesis or vesicle formation to enhance dopamine signaling.
Pretreatment with omega-3 fatty acids before TBI was also shown to prevent losses in brain derived neurotrophic factor, synapsin I, and cAMP response element-binding protein, as well as reduce oxidative stress [35]. In addition, there was a cognitive benefit in rats treated with omega-3 fatty acids: rats treated with omega-3 fatty acid had reduced TBI induced learning deficits. As shown by previous studies and the current report, omega-3 fatty acid enhances dopamine signaling and synaptogenesis. These may be possible mechanisms of cognitive enhancement by omega-3 fatty acid in the setting of TBI.
Attenuation of the striatal dopamine neurotransmission deficit by fish oil may also be associated with the acute neuroprotective effects of omega-3 fatty acids. Several neuroprotective mechanisms of omega-3 fatty acids have been studied including their roles as antioxidants, modulation of inflammatory signaling, and effects on gene expression [6]. In experimental spinal cord injury, dietary DHA or intravenous DHA injection can reduce markers of lipid, protein, and RNA/DNA oxidation, as well as increase neuronal and oligodendrocyte survival [19,21]. Based on these results showing reduction in oxidative stress with DHA treatment, the mechanism of neuroprotection by DHA may be through increases in activity of antioxidant enzymes. Superoxide dismutase activity level was increased whereas xanthine oxidase and nitric oxidase levels were decreased after dietary omega-3 fatty acid treatment in rats [29]. Also, the activity of glutathione peroxidase and glutathione increased in rat hippocampal cultures treated with DHA [33]
Omega-3 fatty acids may also be beneficial in attenuating dopamine neurotransmission deficits by reducing inflammation and changes due to inflammation. Markers of axonal injury and microglia were reduced with DHA treatment in spinal cord injury, and animals showed improvements in motor function [19]. In addition, proinflammatory cytokine IL-6 and inflammatory cell infiltration was reduced by DHA treatment in ischemic brain injury [27]. Also, DHA can serve as a precursor to neuroprotectin D-1 (NPD1) a mediator which has anti-inflammatory and pro-survival signaling functions [6]. NPD-1 administration into cerebral ventricles in an ischemic model reduced neutrophil infiltration, proinflammatory gene signaling, and infarct size [24]. This proinflammatory effect after injury was also reversed by administration of DHA, suggesting that increased DHA supplementation may be neuroprotective by increasing NPD-1 signaling. In addition, intravenous infusion of DHA has been shown to reduce infarct volume and improve behavioral measures [7]. Recent evidences showed that both preinjury [5] and postinjury treatment [4,25] with omega-3 fatty acid for rats subjected to TBI provide neuroprotection. Rats treated with omega-3 fatty acid had reduced markers of injury such as beta amyloid precursor protein, CD68, and caspase-3, supporting the therapeutic potential of omega-3 fatty acid in TBI.
Conclusions
Various mechanisms of omega-3 fatty acid’s protective action in brain injury have been previously proposed. Dopamine neurotransmission is important for behavioral function, and previous research on TBI demonstrated deficits in dopamine synthesis and release. By utilizing microdialysis and HPLC, we demonstrated here that TBI induced deficit in potassium evoked dopamine release can be reversed by 7 day oral fish oil treatment. Future studies are needed to elucidate the mechanism of omega-3 fatty acid increasing striatal dopamine release post TBI.
Highlights.
-Traumatic brain injury induces deficits in striatal dopamine release in rats.
-7 day fish oil treatment of rats by oral gavage induces recovery of striatal dopamine release.
-7 day fish oil treatment does not affect striatal dopamine metabolite levels.
Acknowledgments
Supported by NIH grants: 1F30NS067731-01, 5R01NS060672-02
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agut J, Ortiz JA, Wurtman RJ. Cytidine (5′)diphosphocholine modulates dopamine K(+)-evoked release in striatum measured by microdialysis. Ann N Y Acad Sci. 2000;920:332–5. doi: 10.1111/j.1749-6632.2000.tb06944.x. [DOI] [PubMed] [Google Scholar]
- 2.Aïd S, Vancassel S, Poumès-Ballihaut C, Chalon S, Guesnet P, Lavialle M. Effect of a diet-induced n-3 PUFA depletion on cholinergic parameters in the rat hippocampus. J Lipid Res. 2003;44:1545–51. doi: 10.1194/jlr.M300079-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Aïd S, Vancassel S, Linard A, Lavialle M, Guesnet P. Dietary docosahexaenoic acid [22: 6(n-3)] as a phospholipid or a triglyceride enhances the potassium chloride-evoked release of acetylcholine in rat hippocampus. J Nutr. 2005;135:1008–13. doi: 10.1093/jn/135.5.1008. [DOI] [PubMed] [Google Scholar]
- 4.Bailes JE, Mills JD. Docosahexaenoic acid reduces traumatic axonal injury in a rodent head injury model. J Neurotrauma. 2010;27:1617–24. doi: 10.1089/neu.2009.1239. [DOI] [PubMed] [Google Scholar]
- 5.Bailes JE, Mills JD, Hadley K. Dietary Supplementation with the Omega-3 Fatty Acid Docosahexaenoic Acid in Traumatic Brain Injury? Neurosurgery. 2011 Jan 5; doi: 10.1227/NEU.0b013e3181ff692b. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Bazan NG. Neuroprotectin D1-mediated anti-inflammatory and survival signaling in stroke, retinal degenerations, and Alzheimer’s disease. J Lipid Res. 2009 Apr;50(Suppl):S400–5. doi: 10.1194/jlr.R800068-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belayev L, Khoutorova L, Atkins KD, Bazan NG. Robust docosahexaenoic acid-mediated neuroprotection in a rat model of transient, focal cerebral ischemia. Stroke. 2009 Sep;40(9):3121–6. doi: 10.1161/STROKEAHA.109.555979. Epub 2009 Jun 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bousquet M, Saint-Pierre M, Julien C, Salem N, Cicchetti F, Calon F. Beneficial effects of dietary omega-3 polyunsaturated fatty acid on toxin-induced neuronal degeneration in an animal model of Parkinson’s disease. FASEB J. 2008;22:1213–25. doi: 10.1096/fj.07-9677com. [DOI] [PubMed] [Google Scholar]
- 9.Bousquet M, Gibrat C, Saint-Pierre M, Julien C, Calon F, Cicchetti F. Prog Neuropsychopharmacol Biol Psychiatry. 2009 Nov 13;33(8):1401–8. doi: 10.1016/j.pnpbp.2009.07.018. Epub 2009 Jul 24. Modulation of brain-derived neurotrophic factor as a potential neuroprotective mechanism of action of omega-3 fatty acids in a parkinsonian animal model.
- 10.Cansev M, Wurtman RJ, Sakamoto T, Ulus IH. Oral administration of circulating precursors for membrane phosphatides can promote the synthesis of new brain synapses. Alzheimers Dement. 2008;4(1 Suppl 1):S153–68. doi: 10.1016/j.jalz.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi-Kwon S, Park KA, Lee HJ, Park MS, Lee JH, Jeon SE, et al. Temporal changes in cerebral antioxidant enzyme activities after ischemia and reperfusion in a rat focal brain ischemia model: effect of dietary fish oil. Brain Res Dev Brain Res. 2004;152:11–8. doi: 10.1016/j.devbrainres.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Corrigan FM, Horrobin DF, Skinner ER, Besson JA, Cooper MB. Abnormal content of n-6 and n-3 long-chain unsaturated fatty acids in the phosphoglycerides and cholesterol esters of parahippocampal cortex from Alzheimer’s disease patients and its relationship to acetyl CoA content. Int J Biochem Cell Biol. 1998;30:197–207. doi: 10.1016/s1357-2725(97)00125-8. [DOI] [PubMed] [Google Scholar]
- 13.Dangour AD, Allen E, Elbourne D, Fasey N, Fletcher AE, Hardy P, Holder GE, Knight R, Letley L, Richards M, Uauy R. Effect of 2-y n-3 long-chain polyunsaturated fatty acid supplementation on cognitive function in older people: a randomized, double-blind, controlled trial. Am J Clin Nutr. 2010 Jun;91(6):1725–32. doi: 10.3945/ajcn.2009.29121. Epub 2010 Apr 21. [DOI] [PubMed] [Google Scholar]
- 14.Delattre AM, Kiss A, Szawka RE, Anselmo-Franci JA, Bagatini PB, Xavier LL, Rigon P, Achaval M, Iagher F, de David C, Marroni NA, Ferraz AC. Neurosci Res. 2010 Mar;66(3):256–64. doi: 10.1016/j.neures.2009.11.006. Epub 2009 Nov 24. Evaluation of chronic omega-3 fatty acids supplementation on behavioral and neurochemical alterations in 6-hydroxydopamine-lesion model of Parkinson’s disease.
- 15.Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–62. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- 16.Dixon CE, Ma X, Marion DW. Reduced evoked release of acetylcholine in the rodent neocortex following traumatic brain injury. Brain Res. 1997;749:127–30. doi: 10.1016/s0006-8993(96)01310-8. [DOI] [PubMed] [Google Scholar]
- 17.Favrelière S, Perault MC, Huguet F, De Javel D, Bertrand N, Piriou A, et al. DHA-enriched phospholipid diets modulate age-related alterations in rat hippocampus. Neurobiol Aging. 2003;24:233–43. doi: 10.1016/s0197-4580(02)00064-7. [DOI] [PubMed] [Google Scholar]
- 18.Green D, Barreres L, Borensztajn J, Kaplan P, Reddy MN, Rovner R, Simon H. A double-blind, placebo-controlled trial of fish oil concentrate (MaxEpa) in stroke patients. Stroke. 1985 Jul-Aug;16(4):706–9. doi: 10.1161/01.str.16.4.706. [DOI] [PubMed] [Google Scholar]
- 19.Huang WL, King VR, Curran OE, Dyall SC, Ward RE, Lal N, et al. A combination of intravenous and dietary docosahexaenoic acid significantly improves outcome after spinal cord injury. Brain. 2007;130:3004–19. doi: 10.1093/brain/awm223. [DOI] [PubMed] [Google Scholar]
- 20.Kimura M. Role of basal ganglia in behavioral learning. Neurosci Res. 1995 Jul;22(4):353–8. doi: 10.1016/0168-0102(95)00914-f. [DOI] [PubMed] [Google Scholar]
- 21.King VR, Huang WL, Dyall SC, Curran OE, Priestley JV, Michael-Titus AT. Omega-3 fatty acids improve recovery, whereas omega-6 fatty acids worsen outcome, after spinal cord injury in the adult rat. J Neurosci. 2006;26:4672–80. doi: 10.1523/JNEUROSCI.5539-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kodas E, Vancassel S, Lejeune B, Guilloteau D, Chalon S. Reversibility of n-3 fatty acid deficiency-induced changes in dopaminergic neurotransmission in rats: critical role of developmental stage. J Lipid Res. 2002;43:1209–19. [PubMed] [Google Scholar]
- 23.Korchounov AM. Role of D1 and D2 receptors in the regulation of voluntary movements. Bull Exp Biol Med. 2008 Jul;146(1):14–7. doi: 10.1007/s10517-008-0197-0. [DOI] [PubMed] [Google Scholar]
- 24.Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–17. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 25.Mills JD, Bailes JE, Sedney CL, Hutchins H, Sears B. Omega-3 fatty acid supplementation and reduction of traumatic axonal injury in a rodent head injury model. J Neurosurg. 2011 Jan;114(1):77–84. doi: 10.3171/2010.5.JNS08914. [DOI] [PubMed] [Google Scholar]
- 26.Mura A, Feldon J. Spatial learning in rats is impaired after degeneration of the nigrostriatal dopaminergic system. Mov Disord. 2003 Aug;18(8):860–71. doi: 10.1002/mds.10472. [DOI] [PubMed] [Google Scholar]
- 27.Pan HC, Kao TK, Ou YC, Yang DY, Yen YJ, Wang CC, et al. Protective effect of docosahexanoic acid against brain injury in ischemic rats. J Nutr Biochem. 2009;20:715–25. doi: 10.1016/j.jnutbio.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 28.Peet M. Eicosapentaenoic acid in the treatment of schizophrenia and depression: rationale and preliminary double-blind clinical trial results. Prostaglandins Leukot Essent Fatty Acids. 2003;69:477–85. doi: 10.1016/j.plefa.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 29.Sarsilmaz M, Songur A, Ozyurt H, Kuş I, Ozen OA, Ozyurt B, et al. Potential role of dietary omega-3 essential fatty acids on some oxidant/antioxidant parameters in rats’ corpus striatum. Prostaglandins Leukot Essent Fatty Acids. 2003;69:253–9. doi: 10.1016/s0952-3278(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 30.Shin SS, Bray ER, Zhang CQ, Dixon CE. Traumatic brain injury reduces striatal tyrosine hydroxylase activity and potassium-evoked dopamine release in rats. Brain Res. 2010 doi: 10.1016/j.brainres.2010.10.096. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanriover G, Seval-Celik Y, Ozsoy O, Akkoyunlu G, Savcioglu F, Hacioglu G, Demir N, Agar A. The effects of docosahexaenoic acid on glial derived neurotrophic factor and neurturin in bilateral rat model of Parkinson’s disease. Folia Histochem Cytobiol. 2010 Sep 30;48(3):434–41. doi: 10.2478/v10042-010-0047-6. [DOI] [PubMed] [Google Scholar]
- 32.Wainwrite PE. Do essential fatty acids play a role in brain and behavioral development? Neurosci Biobehav Rev. 1992;16:193–205. doi: 10.1016/s0149-7634(05)80180-0. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Zhao X, Mao ZY, Wang XM, Liu ZL. Neuroprotective effect of docosahexaenoic acid on glutamate-induced cytotoxicity in rat hippocampal cultures. Neuroreport. 2003 Dec 19;14(18):2457–61. doi: 10.1097/00001756-200312190-00033. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Pooler AM, Albrecht MA, Wurtman RJ. Dietary uridine-5′-monophosphate supplementation increases potassium-evoked dopamine release and promotes neurite outgrowth in aged rats. J Mol Neurosci. 2005;27:137–45. doi: 10.1385/JMN:27:1:137. [DOI] [PubMed] [Google Scholar]
- 35.Wu A, Ying Z, Gomez-Pinilla F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J Neurotrauma. 2004;21:1457–67. doi: 10.1089/neu.2004.21.1457. [DOI] [PubMed] [Google Scholar]
- 36.Wurtman RJ, Cansev M, Sakamoto T, Ulus IH. Use of phosphatide precursors to promote synaptogenesis. Annu Rev Nutr. 2009;29:59–87. doi: 10.1146/annurev-nutr-080508-141059. [DOI] [PubMed] [Google Scholar]
- 37.Zimmer L, Hembert S, Durand G, Breton P, Guilloteau D, Besnard JC, et al. Chronic n-3 polyunsaturated fatty acid diet-deficiency acts on dopamine metabolism in the rat frontal cortex: a microdialysis study. Neurosci Lett. 1998;240:177–81. doi: 10.1016/s0304-3940(97)00938-5. [DOI] [PubMed] [Google Scholar]
- 38.Zimmer L, Delpal S, Guilloteau D, Aïoun J, Durand G, Chalon S. Chronic n-3 polyunsaturated fatty acid deficiency alters dopamine vesicle density in the rat frontal cortex. Neurosci Lett. 2000 Apr 21;284(1-2):25–8. doi: 10.1016/s0304-3940(00)00950-2. [DOI] [PubMed] [Google Scholar]
- 39.Zimmer L, Vancassel S, Cantagrel S, Breton P, Delamanche S, Guilloteau D, Durand G, Chalon S. The dopamine mesocorticolimbic pathway is affected by deficiency in n-3 polyunsaturated fatty acids. Am J Clin Nutr. 2002 Apr;75(4):662–7. doi: 10.1093/ajcn/75.4.662. [DOI] [PubMed] [Google Scholar]