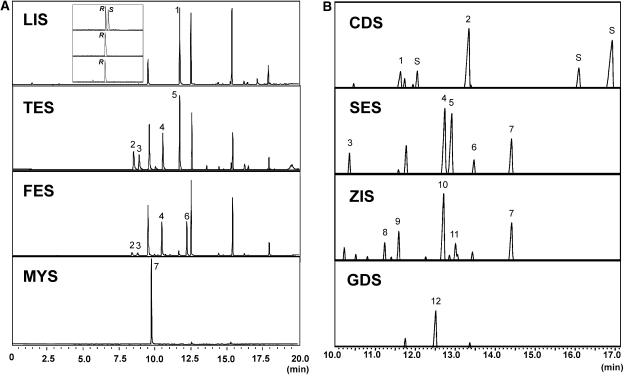
Figure 5.
Identification of the product of the eight basil TPSs. Gas chromatographic separation of products synthesized in vitro by monoterpene and sesquiterpene synthase overexpressed in E. coli. A, Monoterpene synthases LIS, TES, FES, and MYS. Reaction products were obtained by SPME from GPP as a substrate. Peak 1, Linalool; 2, α-pinene; 3, unidentified monoterpene; 4, limonene; 5, terpinolene; 6, fenchol; and 7, β-myrcene. Each compound was identified with comparisons of retention times and mass spectra of authentic standards. The unlabeled peaks are non-terpenoid volatiles also found in control E. coli extracts. Top (inset), GC analysis for (R)-linalool and (S)-linalool stereoisomers on a chiral column. The top trace shows separation of a racemic linalool standard mixture with the (R)-linalool peak labeled R and the (S)-linalool peak labeled S; the middle trace shows linalool extracted from basil glands; the bottom trace shows linalool extracted from the in vitro enzyme assay of E. coli-produced LIS. B, Sesquiterpene synthases CDS, SES, ZIS, and GDS. Reaction products were obtained by SPME from FPP as a substrate. Products were identified with comparisons of retention times and mass spectra of authentic standards. Peak 1, Muurola 3,5-diene; 2, γ-cadinene; 3, β-elemene; 4, β-selinene; 5, α-selinene; 6, epi-α-selinene; 7, nerolidol; 8, α-bergamotene; 9, β-farnesene; 10, α-zingiberene; 11, β-bisabolene; 12, germacrene D. S, Unidentified sesquiterpenes.