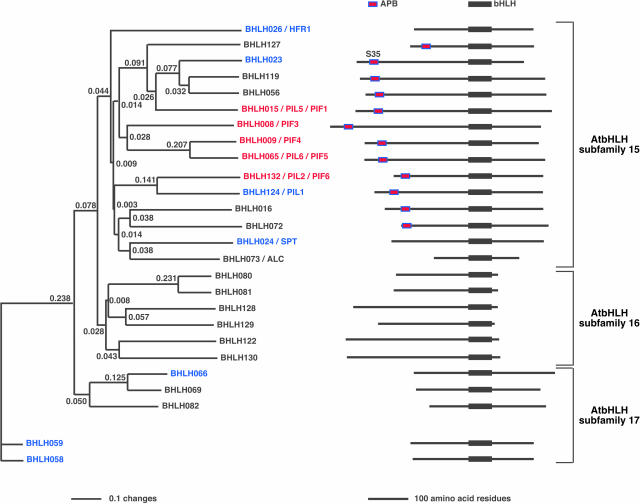
Figure 2.
Members of the PIF3 Group of AtbHLH Proteins Contain a Conserved Sequence in their N-Terminal Domains.
Neighbor-joining phylogenetic tree using full-length amino acid sequences of proteins closely related to PIF3. The tree was constructed with PAUP 4.0 software using an alignment (MultiAlin; Corpet, 1988) of predicted full-length amino acid sequences for each protein. All proteins are identified by their generic names and grouped in their subfamilies (Bailey et al., 2003), and other synonym/s are indicated. Twelve of the full-length AtbHLH proteins from different subfamilies have been tested for interactions with phyB using in vitro coimmunoprecipitation assays. Seven of the tested proteins did not interact (in blue) with phyB, whereas five show interactions (in red) specifically with the active Pfr form of phyB. Four AtbHLH proteins previously tested for interactions with phyB are HFR1 (Fairchild et al., 2000), PIF3 (Ni et al., 1998), PIF4 (Huq and Quail, 2002), and PIF1 (also known as PIL5; Yamashino et al., 2003; Huq et al., 2004). We propose two new names, PIF5 (old name, PIL6; Yamashino et al., 2003) and PIF6 (old name, PIL2; Yamashino et al., 2003) for the new phyB interacting factors to reflect their molecular activity. On the right, we show stick diagrams of the full-length proteins aligned at their bHLH domains. The presence of the APB consensus sequence in 12 AtbHLH proteins in subfamily 15 is indicated, including the S35 present in BHLH023, instead of the invariant G. The branch lengths are proportional to the indicated distance values (changes) between sequences. SPT (Heisler et al., 2001); ALC, ALCATRAZ (Rajani and Sundaresan, 2001). Proteins that interact with phyB (Pfr) are in red, and those that were tested but do not interact are in blue.